DMRT1 repression using a novel approach to genetic manipulation induces testicular dysgenesis in human fetal gonads
- PMID: 30272154
- PMCID: PMC6195803
- DOI: 10.1093/humrep/dey289
DMRT1 repression using a novel approach to genetic manipulation induces testicular dysgenesis in human fetal gonads
Abstract
Study question: Does loss of DMRT1 in human fetal testis alter testicular development and result in testicular dysgenesis?
Summary answer: DMRT1 repression in human fetal testis alters the expression of key testicular and ovarian determining genes, and leads to focal testicular dysgenesis.
What is known already: Testicular dysgenesis syndrome (TDS) is associated with common testicular disorders in young men, but its etiology is unknown. DMRT1 has been shown to play a role in the regulation of sex differentiation in the vertebrate gonad. Downregulation of DMRT1 in male mice results in trans-differentiation of Sertoli cells into granulosa (FOXL2+) cells resulting in an ovarian gonadal phenotype.
Study design, size, duration: To determine the effect of DMRT1 repression on human fetal testes, we developed a novel system for genetic manipulation, which utilizes a Lentivral delivered miRNA during short-term in vitro culture (2 weeks). A long-term (4-6 weeks) ex vivo xenograft model was used to determine the subsequent effects of DMRT1 repression on testicular development and maintenance. We included first and second-trimester testis tissue (8-20 weeks gestation; n = 12) in the study.
Participants/materials, setting, methods: Human fetal testes were cultured in vitro and exposed to either of two DMRT1 miRNAs (miR536, miR641), or to scrambled control miRNA, for 24 h. This was followed by a further 14 days of culture (n = 3-4), or xenografting (n = 5) into immunocompromised mice for 4-6 weeks. Tissues were analyzed by histology, immunohistochemistry, immunofluorescence and quantitative RT-PCR. Endpoints included histological evaluation of seminiferous cord integrity, mRNA expression of testicular, ovarian and germ cell genes, and assessment of cell number and protein expression for proliferation, apoptosis and pluripotency factors. Statistical analysis was performed using a linear mixed effect model.
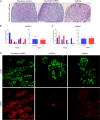
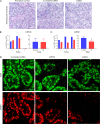
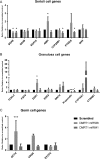
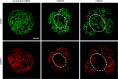
Main results and the role of chance: DMRT1 repression (miR536/miR641) resulted in a loss of DMRT1 protein expression in a sub-population of Sertoli cells of first trimester (8-11 weeks gestation) human fetal testis; however, this did not affect the completion of seminiferous cord formation or morphological appearance. In second-trimester testis (12-20 weeks gestation), DMRT1 repression (miR536/miR641) resulted in disruption of seminiferous cords with absence of DMRT1 protein expression in Sertoli (SOX9+) cells. No differences in proliferation (Ki67+) were observed and apoptotic cells (CC3+) were rare. Expression of the Sertoli cell associated gene, SOX8, was significantly reduced (miR536, 34% reduction, P = 0.031; miR641 36% reduction, P = 0.026), whilst SOX9 expression was unaffected. Changes in expression of AMH (miR536, 100% increase, P = 0.033), CYP26B1 (miR641, 38% reduction, P = 0.05) and PTGDS (miR642, 30% reduction, P = 0.0076) were also observed. Amongst granulosa cell associated genes, there was a significant downregulation in R-spondin 1 expression (miR536, 76% reduction, P < 0.0001; miR641, 49% reduction, P = 0.046); however, there were no changes in expression of the granulosa cell marker, FOXL2. Analysis of germ cell associated genes demonstrated a significant increase in the expression of the pluripotency gene OCT4 (miR536, 233%, P < 0.001). We used the xenograft system to investigate the longer-term effects of seminiferous cord disruption via DMRT1 repression. As was evident in vitro for second-trimester samples, DMRT1 repression resulted in focal testicular dysgenesis similar to that described in adults with TDS. These dysgenetic areas were devoid of germ cells, whilst expression of FOXL2 within the dysgenetic areas, indicated trans-differentiation from a male (Sertoli cell) to female (granulosa cell) phenotype.
Limitations, reasons for caution: Human fetal testis tissue is a limited resource; however, we were able to demonstrate significant effects of DMRT1 repression on the expression of germ and somatic cell genes, in addition to the induction of focal testicular dysgenesis, using these limited samples. In vitro culture may not reflect all aspects of human fetal testis development and function; however, the concurrent use of the xenograft model which represents a more physiological system supports the validity of the in vitro findings.
Wider implications of the findings: Our findings have important implications for understanding the role of DMRT1 in human testis development and in the origin of testicular dysgenesis. In addition, we provide validation of a novel system that can be used to determine the effects of repression of genes that have been implicated in gonadal development and associated human reproductive disorders.
Study funding/competing interest(s): This project was funded by a Wellcome Trust Intermediate Clinical Fellowship (Grant No. 098522) awarded to RTM. LBS was supported by MRC Programme Grant MR/N002970/1. RAA was supported by MRC Programme Grant G1100357/1. RMS was supported by MRC Programme Grant G33253. This work was undertaken in the MRC Centre for Reproductive Health which is funded by the MRC Centre grant MR/N022556/1. The funding bodies had no input into the conduct of the research or the production of this manuscript. The authors have declared no conflicts of interest.
Figures



Similar articles
-
Dysregulation of FGFR signalling by a selective inhibitor reduces germ cell survival in human fetal gonads of both sexes and alters the somatic niche in fetal testes.Hum Reprod. 2019 Nov 1;34(11):2228-2243. doi: 10.1093/humrep/dez191. Hum Reprod. 2019. PMID: 31734698 Free PMC article.
-
Ex vivo culture of human fetal gonads: manipulation of meiosis signalling by retinoic acid treatment disrupts testis development.Hum Reprod. 2015 Oct;30(10):2351-63. doi: 10.1093/humrep/dev194. Epub 2015 Aug 6. Hum Reprod. 2015. PMID: 26251460 Free PMC article.
-
Dynamics of the transcriptional landscape during human fetal testis and ovary development.Hum Reprod. 2020 May 1;35(5):1099-1119. doi: 10.1093/humrep/deaa041. Hum Reprod. 2020. PMID: 32412604
-
[Pathogenesis of neoplastic changes in germ cells as a developmental aspect].Pediatr Endocrinol Diabetes Metab. 2007;13(1):37-42. Pediatr Endocrinol Diabetes Metab. 2007. PMID: 17493405 Review. Polish.
-
Early manifestations of testicular dysgenesis in children: pathological phenotypes, karyotype correlations and precursor stages of tumour development.APMIS. 2003 Jan;111(1):12-23; discussion 23-4. doi: 10.1034/j.1600-0463.2003.1110104.x. APMIS. 2003. PMID: 12760349 Review.
Cited by
-
Disorders of Sex Development-Novel Regulators, Impacts on Fertility, and Options for Fertility Preservation.Int J Mol Sci. 2020 Mar 26;21(7):2282. doi: 10.3390/ijms21072282. Int J Mol Sci. 2020. PMID: 32224856 Free PMC article. Review.
-
Zebrafish as an emerging model to study gonad development.Comput Struct Biotechnol J. 2020 Sep 5;18:2373-2380. doi: 10.1016/j.csbj.2020.08.025. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32994895 Free PMC article. Review.
-
DMRT1 regulates human germline commitment.Nat Cell Biol. 2023 Oct;25(10):1439-1452. doi: 10.1038/s41556-023-01224-7. Epub 2023 Sep 14. Nat Cell Biol. 2023. PMID: 37709822 Free PMC article.
-
Monogenic causes of non-obstructive azoospermia: challenges, established knowledge, limitations and perspectives.Hum Genet. 2021 Jan;140(1):135-154. doi: 10.1007/s00439-020-02112-y. Epub 2020 Jan 18. Hum Genet. 2021. PMID: 31955275 Review.
-
Trans-ethnic GWAS meta-analysis of idiopathic spermatogenic failure highlights the immune-mediated nature of Sertoli cell-only syndrome.Commun Biol. 2025 Apr 5;8(1):571. doi: 10.1038/s42003-025-08001-2. Commun Biol. 2025. PMID: 40188177 Free PMC article.
References
-
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 2009;327:301–312. - PubMed
-
- Bayne RA, Martins da Silva SJ, Anderson RA. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod 2004;10:373–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

