Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro
- PMID: 30272303
- PMCID: PMC6196606
- DOI: 10.3892/or.2018.6723
Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro
Abstract
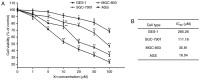
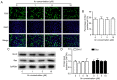
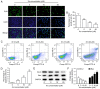
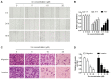
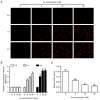
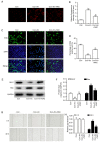
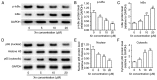
Xanthohumol (Xn), a prenylated flavonoid isolated from Hops (Humulus lupulus L.), has demonstrated potent anticancer activity in multiple types of cancer. However, the effect of Xn on gastric cancer (GC) remains unknown. The aim of the present study was to investigate the effect of Xn on GC cell proliferation, apoptosis and metastasis. It was observed that Xn decreased the viability of GC cells, with very low or no toxicity to normal gastric epithelial cells GES‑1 at a concentration of 1‑100 µM. The proliferation of AGS cells was inhibited by Xn, as indicated by the decreased number of EdU‑positive cells. Xn treatment increased the number of apoptotic cells, downregulated the expression of Bcl‑2 and upregulated the expression of Bax, suggesting induction of apoptosis. The results from the wound healing and Transwell assays indicated that Xn suppressed AGS cell metastasis. Moreover, Xn induced reactive oxygen species (ROS) overproduction and inhibited nuclear factor (NF)‑κB signaling in AGS cells, which was reversed by the ROS inhibitor N‑acetylcysteine (NAC). NAC suppressed the effect of Xn on the proliferation, apoptosis and metastasis of AGS cells. Taken together, these results suggest that Xn exerts anticancer effects against GC via induction of ROS production and subsequent inhibition of NF‑κB signaling. Therefore, Xn may be a promising candidate treatment against GC progression.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

