Standardized Measurement of Nasal Membrane Transepithelial Potential Difference (NPD)
- PMID: 30272672
- PMCID: PMC6235174
- DOI: 10.3791/57006
Standardized Measurement of Nasal Membrane Transepithelial Potential Difference (NPD)
Abstract
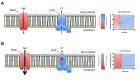
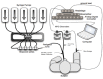
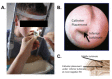
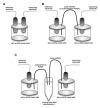
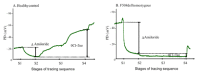
We describe a standardized measurement of nasal potential difference (NPD). In this technique, cystic fibrosis transmembrane conductance regulator (CFTR) and the epithelial sodium channel (ENaC) function are monitored by the change in voltage across the nasal epithelium after the superfusion of solutions that modify ion channel activity. This is enabled by the measurement of the potential difference between the subcutaneous compartment and the airway epithelium in the nostril, utilizing a catheter in contact with the inferior nasal turbinate. The test allows the measurement of the stable baseline voltage and the successive net voltage changes after perfusion of 100 µM amiloride, an inhibitor of Na+ reabsorption in Ringer's solution; a chloride-free solution containing amiloride to drive chloride secretion and 10 µM isoproterenol in a chloride-free solution with amiloride to stimulate the cyclic adenosine monophosphate (cAMP)-dependent chloride conductance related to CFTR. This technique has the advantage of demonstrating the electrophysiological properties of two key components establishing the hydration of the airway surface liquid of the respiratory epithelium, ENaC, and CFTR. Therefore, it is a useful research tool for phase 2 and proof of concept trials of agents that target CFTR and ENaC activity for the treatment of cystic fibrosis (CF) lung disease. It is also a key follow-up procedure to establish CFTR dysfunction when genetic testing and sweat testing are equivocal. Unlike sweat chloride, the test is relatively more time consuming and costly. It also requires operator training and expertise to conduct the test effectively. Inter- and intra-subject variability has been reported in this technique especially in young or uncooperative subjects. To assist with this concern, interpretation has been improved through a recently validated algorithm.
References
-
- Rosenstein B. What is a Cystic Fibrosis Diagnosis? Clinics in Chest Medicine. 1998;19:423–441. - PubMed
-
- Rosenstein B, Cutting GR. The Diagnosis of Cystic Fibrosis: A Consensus Statement: Cystic Fibrosis Foundation Consensus Panel. The Journal of Pediatrics. 1998;132:589–595. - PubMed
-
- Farrell PM, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. The Journal of Pediatrics. 2017;181:S4–S15. - PubMed
-
- Mesbahi M, et al. Changes of CFTR functional measurements and clinical improvements in cystic fibrosis patients with non-p.Gly551Asp gating mutations treated with ivacaftor. Journal of Cystic Fibrosis. 2017;16:45–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical