Photodynamic Inactivation of Herpes Simplex Viruses
- PMID: 30274257
- PMCID: PMC6213367
- DOI: 10.3390/v10100532
Photodynamic Inactivation of Herpes Simplex Viruses
Abstract
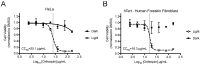
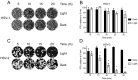
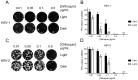
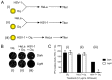
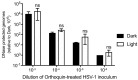
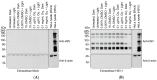
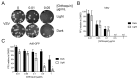
Herpes simplex virus (HSV) infections can be treated with direct acting antivirals like acyclovir and foscarnet, but long-term use can lead to drug resistance, which motivates research into broadly-acting antivirals that can provide a greater genetic barrier to resistance. Photodynamic inactivation (PDI) employs a photosensitizer, light, and oxygen to create a local burst of reactive oxygen species that inactivate microorganisms. The botanical plant extract OrthoquinTM is a powerful photosensitizer with antimicrobial properties. Here we report that Orthoquin also has antiviral properties. Photoactivated Orthoquin inhibited herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) infection of target cells in a dose-dependent manner across a broad range of sub-cytotoxic concentrations. HSV inactivation required direct contact between Orthoquin and the inoculum, whereas pre-treatment of target cells had no effect. Orthoquin did not cause appreciable damage to viral capsids or premature release of viral genomes, as measured by qPCR for the HSV-1 genome. By contrast, immunoblotting for HSV-1 antigens in purified virion preparations suggested that higher doses of Orthoquin had a physical impact on certain HSV-1 proteins that altered protein mobility or antigen detection. Orthoquin PDI also inhibited the non-enveloped adenovirus (AdV) in a dose-dependent manner, whereas Orthoquin-mediated inhibition of the enveloped vesicular stomatitis virus (VSV) was light-independent. Together, these findings suggest that the broad antiviral effects of Orthoquin-mediated PDI may stem from damage to viral attachment proteins.
Keywords: HSV-1; HSV-2; antiviral; natural product; photodynamic inactivation; plaque assay.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Fields B.N., Knipe D.M., Howley P.M. Fields Virology. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

