Molecular Scoring of Hepatocellular Carcinoma for Predicting Metastatic Recurrence and Requirements of Systemic Chemotherapy
- PMID: 30274313
- PMCID: PMC6210853
- DOI: 10.3390/cancers10100367
Molecular Scoring of Hepatocellular Carcinoma for Predicting Metastatic Recurrence and Requirements of Systemic Chemotherapy
Abstract
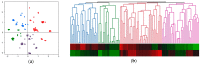
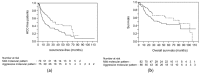
Hepatocellular carcinoma (HCC) causes one of the most frequent cancer-related deaths; an HCC subset shows rapid progression that affects survival. We clarify molecular features of aggressive HCC, and establish a molecular scoring system that predicts metastasis after curative treatment. In total, 125 HCCs were examined for TP53, CTNNB1, and TERT promoter mutation, methylation of 8 tumor suppressor genes, and 3 repetitive DNA sequences to estimate promoter hypermethylation and global hypomethylation. A fractional allelic loss (FAL) was calculated to represent chromosomal instability through microsatellite analysis. Molecular subclasses were determined using corresponding and hierarchical clustering analyses. Next, twenty-five HCC patients who underwent liver transplantation were analyzed for associations between molecular characteristics and metastatic recurrence; survival analyses were validated using a publicly available dataset of 376 HCC cases from the Cancer Genome Atlas (TCGA). An HCC subtype characterized by TP53 mutation, high FAL, and global hypomethylation was associated with aggressive tumor characteristics, like vascular invasion; CTNNB1 mutation was a feature of the less-progressive phenotype. A number of molecular risk factors, including TP53 mutation, high FAL, significant global hypomethylation, and absence of CTNNB1 mutation, were noted to predict shorter recurrence-free survival in patients who underwent liver transplantation (p = 0.0090 by log-rank test). These findings were validated in a cohort of resected HCC cases from TCGA (p = 0.0076). We concluded that molecular risks determined by common genetic and epigenetic alterations could predict metastatic recurrence after curative treatments, and could be a marker for considering systemic therapy for HCC patients.
Keywords: chromosomal alteration; hepatocellular carcinoma; liver transplantation; methylation; molecular subclass; mutation; recurrence; systemic chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
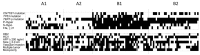

Similar articles
-
Unique association between global DNA hypomethylation and chromosomal alterations in human hepatocellular carcinoma.PLoS One. 2013 Sep 2;8(9):e72312. doi: 10.1371/journal.pone.0072312. eCollection 2013. PLoS One. 2013. PMID: 24023736 Free PMC article.
-
Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification.J Hepatol. 2017 Oct;67(4):727-738. doi: 10.1016/j.jhep.2017.05.014. Epub 2017 May 19. J Hepatol. 2017. PMID: 28532995
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
-
Combination of LINE-1 hypomethylation and RASSF1A promoter hypermethylation in serum DNA is a non-invasion prognostic biomarker for early recurrence of hepatocellular carcinoma after curative resection.Neoplasma. 2017;64(5):795-802. doi: 10.4149/neo_2017_519. Neoplasma. 2017. PMID: 28592132
-
Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections.Genomics. 2013 Aug;102(2):74-83. doi: 10.1016/j.ygeno.2013.04.001. Epub 2013 Apr 11. Genomics. 2013. PMID: 23583669 Review.
Cited by
-
Integrative multi-omics analysis reveals a novel subtype of hepatocellular carcinoma with biological and clinical relevance.Front Immunol. 2024 Dec 6;15:1517312. doi: 10.3389/fimmu.2024.1517312. eCollection 2024. Front Immunol. 2024. PMID: 39712016 Free PMC article.
-
Advancements in Artificial Intelligence-Enhanced Imaging Diagnostics for the Management of Liver Disease-Applications and Challenges in Personalized Care.Bioengineering (Basel). 2024 Dec 9;11(12):1243. doi: 10.3390/bioengineering11121243. Bioengineering (Basel). 2024. PMID: 39768061 Free PMC article. Review.
-
Liver damage related to immune checkpoint inhibitors.Hepatol Int. 2019 May;13(3):248-252. doi: 10.1007/s12072-018-9921-7. Epub 2019 Jan 3. Hepatol Int. 2019. PMID: 30607787 Review.
-
Incremental value of radiomics-based heterogeneity to the existing risk criteria in predicting recurrence of hepatocellular carcinoma after liver transplantation.Eur Radiol. 2023 Sep;33(9):6608-6618. doi: 10.1007/s00330-023-09591-3. Epub 2023 Apr 4. Eur Radiol. 2023. PMID: 37012548
-
Geographic and Viral Etiology Patterns of TERT Promoter and CTNNB1 Exon 3 Mutations in Hepatocellular Carcinoma: A Comprehensive Review.Int J Mol Sci. 2025 Mar 22;26(7):2889. doi: 10.3390/ijms26072889. Int J Mol Sci. 2025. PMID: 40243493 Free PMC article. Review.
References
-
- Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

