Prostaglandin E1-Mediated Collateral Recruitment Is Delayed in a Neonatal Rat Stroke Model
- PMID: 30274381
- PMCID: PMC6213314
- DOI: 10.3390/ijms19102995
Prostaglandin E1-Mediated Collateral Recruitment Is Delayed in a Neonatal Rat Stroke Model
Abstract
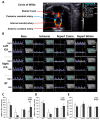
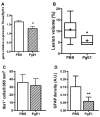
While arterial reflow after a stroke represents an important challenge for better outcomes, it is also very important that sudden recanalization does not produce local oxidative and nitrogen species, deleterious for the brain and more particularly the immature brain. Our objective was to determine whether a supply in prostaglandin (Pg) E1 (Alprostadil), via its action on arterial pressure, might progressively improve cerebral reperfusion in a neonatal stroke model. Arterial blood flow was measured using ultrasonography. Rate-limiting and Pg terminal synthesizing enzymes were evaluated using reverse-transcriptase polymerase chain reaction. Our data suggests that a supply in PgE1 might delay and improve the ipsilateral reperfusion by decreasing thromboxane A synthase-1 gene, the density of reactive astrocytes and lesion volume.
Keywords: Astrocytes; Neonatal ischemia; hemodynamic responses; prostaglandins; thromboxane A synthase-1; ultrasound imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Van Bel F., Dorrepaal C.A., Benders M.J., Zeeuwe P.E., van de Bor M., Berger H.M. Changes in cerebral hemodynamics and oxygenation in the first 24 h after birth asphyxia. Pediatrics. 1993;92:365–372. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

