Glycan recognition at the saliva - oral microbiome interface
- PMID: 30274839
- PMCID: PMC6296888
- DOI: 10.1016/j.cellimm.2018.08.008
Glycan recognition at the saliva - oral microbiome interface
Abstract
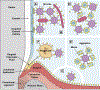
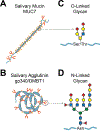
The mouth is a first critical interface where most potentially harmful substances or pathogens contact the host environment. Adaptive and innate immune defense mechanisms are established there to inactivate or eliminate pathogenic microbes that traverse the oral environment on the way to their target organs and tissues. Protein and glycoprotein components of saliva play a particularly important role in modulating the oral microbiota and helping with the clearance of pathogens. It has long been acknowledged that glycobiological and glycoimmunological aspects play a pivotal role in oral host-microbe, microbe-host, and microbe-microbe interactions in the mouth. In this review, we aim to delineate how glycan-mediated host defense mechanisms in the oral cavity support human health. We will describe the role of glycans attached to large molecular size salivary glycoproteins which act as a first line of primordial host defense in the human mouth. We will further discuss how glycan recognition contributes to both colonization and clearance of oral microbes.
Keywords: Evolution; Glycans; Glycobiology; Host defense; Microbial adhesins; Microbiology; Mucins; Oral biology; Saliva; Salivary proteins.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sreebny LM, Saliva in health and disease: an appraisal and update, Int Dent J, 50 (2000) 140–161. - PubMed
-
- Dawes C, Pedersen AM, Villa A, Ekstrom J, Proctor GB, Vissink A, Aframian D, McGowan R, Aliko A, Narayana N, Sia YW, Joshi RK, Jensen SB, Kerr AR, Wolff A, The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI, Arch Oral Biol, 60 (2015) 863–874. - PubMed
-
- Eichwald E, Beiträge zur Chemie der gewebbildenden Substanzen und ihrer Abkömmlinge. I. Über das Mucin, besonders der Weinbergschnecke, Annalen der Chemie und Pharmacie, 134 (1865) 177–211.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

