Ligand binding and heterodimerization with retinoid X receptor α (RXRα) induce farnesoid X receptor (FXR) conformational changes affecting coactivator binding
- PMID: 30275017
- PMCID: PMC6254343
- DOI: 10.1074/jbc.RA118.004652
Ligand binding and heterodimerization with retinoid X receptor α (RXRα) induce farnesoid X receptor (FXR) conformational changes affecting coactivator binding
Abstract
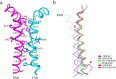
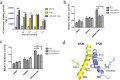
Nuclear receptor farnesoid X receptor (FXR) functions as the major bile acid sensor coordinating cholesterol metabolism, lipid homeostasis, and absorption of dietary fats and vitamins. Because of its central role in metabolism, FXR represents an important drug target to manage metabolic and other diseases, such as primary biliary cirrhosis and nonalcoholic steatohepatitis. FXR and nuclear receptor retinoid X receptor α (RXRα) form a heterodimer that controls the expression of numerous downstream genes. To date, the structural basis and functional consequences of the FXR/RXR heterodimer interaction have remained unclear. Herein, we present the crystal structures of the heterodimeric complex formed between the ligand-binding domains of human FXR and RXRα. We show that both FXR and RXR bind to the transcriptional coregulator steroid receptor coactivator 1 with higher affinity when they are part of the heterodimer complex than when they are in their respective monomeric states. Furthermore, structural comparisons of the FXR/RXRα heterodimers and the FXR monomers bound with different ligands indicated that both heterodimerization and ligand binding induce conformational changes in the C terminus of helix 11 in FXR that affect the stability of the coactivator binding surface and the coactivator binding in FXR. In summary, our findings shed light on the allosteric signal transduction in the FXR/RXR heterodimer, which may be utilized for future drug development targeting FXR.
Keywords: Farnesoid X receptor; Retinoid X receptor; X-ray crystallography; allosteric regulation; allostery; conformational change; metabolism; nuclear receptor; signal transduction; transcription factor.
© 2018 Wang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
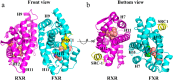




References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

