Bidirectional intraflagellar transport is restricted to two sets of microtubule doublets in the trypanosome flagellum
- PMID: 30275108
- PMCID: PMC6279389
- DOI: 10.1083/jcb.201805030
Bidirectional intraflagellar transport is restricted to two sets of microtubule doublets in the trypanosome flagellum
Abstract
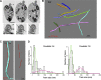
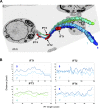
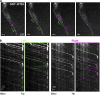
Intraflagellar transport (IFT) is the rapid bidirectional movement of large protein complexes driven by kinesin and dynein motors along microtubule doublets of cilia and flagella. In this study, we used a combination of high-resolution electron and light microscopy to investigate how and where these IFT trains move within the flagellum of the protist Trypanosoma brucei Focused ion beam scanning electron microscopy (FIB-SEM) analysis of trypanosomes showed that trains are found almost exclusively along two sets of doublets (3-4 and 7-8) and distribute in two categories according to their length. High-resolution live imaging of cells expressing mNeonGreen::IFT81 or GFP::IFT52 revealed for the first time IFT trafficking on two parallel lines within the flagellum. Anterograde and retrograde IFT occurs on each of these lines. At the distal end, a large individual anterograde IFT train is converted in several smaller retrograde trains in the space of 3-4 s while remaining on the same side of the axoneme.
© 2018 Bertiaux et al.
Figures



Comment in
-
Can microtubule motors use every available track?J Cell Biol. 2018 Dec 3;217(12):4055-4056. doi: 10.1083/jcb.201810083. Epub 2018 Nov 7. J Cell Biol. 2018. PMID: 30404947 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

