Bone Health and Osteoporosis Management of the Patient With Duchenne Muscular Dystrophy
- PMID: 30275247
- PMCID: PMC6442478
- DOI: 10.1542/peds.2018-0333E
Bone Health and Osteoporosis Management of the Patient With Duchenne Muscular Dystrophy
Abstract
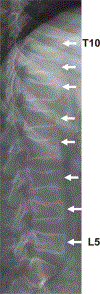
Duchenne muscular dystrophy is associated with an increased risk of bone fragility due to the adverse effects of prolonged glucocorticoid therapy and progressive muscle weakness on bone strength. Osteoporosis manifests clinically as low-trauma long-bone and vertebral fractures (VFs), with VFs frequent, particularly in those treated with glucocorticoid therapy. It is increasingly recognized that bone pain, medical complications of osteoporosis (such as fat embolism syndrome), and the potential for permanent, fracture-induced loss of ambulation can be mitigated with timely bone health surveillance and management. This includes periodic spine radiographs for VF detection because VFs can be asymptomatic in their early phases and thereby go undetected in the absence of monitoring. With this article, we provide a comprehensive review of the following 4 phases of bone health management: (1) bone health monitoring, which is used to identify early signs of compromised bone health; (2) osteoporosis stabilization, which is aimed to mitigate back pain and interrupt the fracture-refracture cycle through bone-targeted therapy; (3) bone health maintenance, which has the goal to preserve the clinical gains realized during the stabilization phase through ongoing bone-targeted therapy; and (4) osteoporosis therapy discontinuation, which places those who are eligible for discontinuation of osteoporosis treatment back on a health monitoring program. In the course of reviewing these 4 phases of management, we will discuss the criteria for diagnosing osteoporosis, along with detailed recommendations for osteoporosis intervention including specific drugs, dose, length of therapy, contraindications, and monitoring of treatment efficacy and safety.
Copyright © 2018 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Ward was a consultant to and participated in clinical trials with Novartis; Dr Weber was a paid consultant for Marathon Pharmaceuticals; and Drs Hadjiyannakis, McMillan, and Noritz have indicated they have no potential conflicts of interest to disclose.
Figures

References
-
- Ma J, McMillan HJ, Karagüzel G, et al. The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int 2017;28(2):597–608 - PubMed
-
- McAdam LC, Rastogi A, Macleod K, Douglas Biggar W. Fat Embolism Syndrome following minor trauma in Duchenne muscular dystrophy. Neuromuscul Disord 2012;22(12):1035–1039 - PubMed
-
- Medeiros MO, Behrend C, King W, Sanders J, Kissel J, Ciafaloni E. Fat embolism syndrome in patients with Duchenne muscular dystrophy. Neurology 2013;80(14):1350–1352 - PubMed
-
- Gordon KE, Dooley JM, Sheppard KM, MacSween J, Esser MJ. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics 2011;127(2). Available at: www.pediatrics.org/cgi/content/full/127/2/e353 - PubMed
-
- Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 2000;20(1):71–74 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

