In vivo effects of mercury (II) on deoxyuridine triphosphate nucleotidohydrolase, DNA polymerase (alpha, beta), and uracil-DNA glycosylase activities in cultured human cells: relationship to DNA damage, DNA repair, and cytotoxicity
- PMID: 3027530
- PMCID: PMC2901161
In vivo effects of mercury (II) on deoxyuridine triphosphate nucleotidohydrolase, DNA polymerase (alpha, beta), and uracil-DNA glycosylase activities in cultured human cells: relationship to DNA damage, DNA repair, and cytotoxicity
Abstract
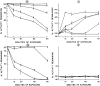
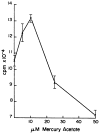
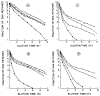
The effect of mercuric acetate on the activities of deoxyuridine triphosphate nucleotidohydrolase (dUTPase), DNA polymerase (alpha, beta), and uracil-DNA glycosylase has been studied in cultured human KB cells. There was a dose- and time-dependent inactivation of both dUTPase and DNA polymerase alpha activities by mercuric acetate. In cells exposed to low concentrations (10 microM) of mercuric acetate, dUTPase was most sensitive to inhibition with 30% of the activity being inhibited after a 1-hr exposure. At higher concentrations or for longer exposure times, DNA polymerase alpha was most sensitive to inhibition with greater than 60% of the activity being inhibited by 25 microM mercuric acetate after a 15-min exposure. There was no inhibition of DNA polymerase beta or uracil-DNA glycosylase activities in cells exposed to 50 microM mercuric acetate for 90 min. In fact, there was a time- and dose-dependent activation of uracil-DNA glycosylase activity with maximum activation occurring in cells exposed to 50 microM mercuric acetate. The inhibition of dUTPase and DNA polymerase alpha activities and the activation of uracil-DNA glycosylase activity correlated with the induction of single-strand breaks in DNA by mercuric acetate and with the decrease in cell viability.
Figures

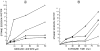
Similar articles
-
Enhancement of methotrexate cytotoxicity by uracil analogues that inhibit deoxyuridine triphosphate nucleotidohydrolase (dUTPase) activity.Adv Exp Med Biol. 1986;195 Pt B:97-104. doi: 10.1007/978-1-4684-1248-2_16. Adv Exp Med Biol. 1986. PMID: 3020931 No abstract available.
-
The role of deoxyuridine triphosphate nucleotidohydrolase, uracil-DNA glycosylase, and DNA polymerase alpha in the metabolism of FUdR in human tumor cells.Mol Pharmacol. 1980 Nov;18(3):513-20. Mol Pharmacol. 1980. PMID: 6110169 No abstract available.
-
Expression of uracil DNA glycosylase (UDG) does not affect cellular sensitivity to thymidylate synthase (TS) inhibition.Eur J Cancer. 2003 Feb;39(3):378-87. doi: 10.1016/s0959-8049(02)00610-x. Eur J Cancer. 2003. PMID: 12565992
-
The nature of enzymes involved in uracil-DNA repair: isoform characteristics of proteins responsible for nuclear and mitochondrial genomic integrity.Curr Protein Pept Sci. 2001 Dec;2(4):335-47. doi: 10.2174/1389203013381044. Curr Protein Pept Sci. 2001. PMID: 12369930 Review.
-
Precluding uracil from DNA.Structure. 1996 Dec 15;4(12):1381-5. doi: 10.1016/s0969-2126(96)00145-1. Structure. 1996. PMID: 8994964 Review.
Cited by
-
Yaravirus brasiliense genomic structure analysis and its possible influence on the metabolism.Genet Mol Biol. 2025 Feb 7;48(1):e20240139. doi: 10.1590/1678-4685-GMB-2024-0139. eCollection 2025. Genet Mol Biol. 2025. PMID: 39918235 Free PMC article.
-
Effects of methyl and inorganic mercury exposure on genome homeostasis and mitochondrial function in Caenorhabditis elegans.DNA Repair (Amst). 2017 Apr;52:31-48. doi: 10.1016/j.dnarep.2017.02.005. Epub 2017 Feb 13. DNA Repair (Amst). 2017. PMID: 28242054 Free PMC article.
-
The L1 retrotranspositional stimulation by particulate and soluble cadmium exposure is independent of the generation of DNA breaks.Int J Environ Res Public Health. 2006 Jun;3(2):121-8. doi: 10.3390/ijerph2006030015. Int J Environ Res Public Health. 2006. PMID: 16823085 Free PMC article.
-
Adaption of Synechococcus sp. IU 625 to growth in the presence of mercuric chloride.Acta Histochem. 2012 Jan;114(1):6-11. doi: 10.1016/j.acthis.2011.01.004. Epub 2011 Mar 15. Acta Histochem. 2012. PMID: 21411123 Free PMC article.
-
Use of genetic toxicology data in U.S. EPA risk assessment: the mercury study report as an example.Environ Health Perspect. 1996 May;104 Suppl 3(Suppl 3):663-73. doi: 10.1289/ehp.96104s3663. Environ Health Perspect. 1996. PMID: 8781402 Free PMC article.
References
-
- Greunweld DW, Cruickshank MK. Effect of methylmercury (II) on the synthesis of deoxyribonucleic acid, ribonucleic acid and protein in HeLa S3 cells. Biochem Pharmacol. 1979;28:661–656. - PubMed
-
- Chao E, Gierthy JF, Frenkel GD. A comparative study of the effects of mercury compounds on cell viability and nucleic acid synthesis in HeLa cells. Biochem Pharmacol. 1984;33:1941–1945. - PubMed
-
- Costa M, Cantoni O, deMars M, Swartzendruber DE. Toxic metals produce an S-phase specific cell block. Res Commun Chem Pathol Pharmacol. 1982;38:405–419. - PubMed
-
- Christie NT, Cantoni O, Sugiyama M, Cattabeni F, Costa M. Differences in the effects of Hg(II) on DNA repair induced in Chinese hamster ovary cells by ultraviolet or X-rays. Mol Pharmacol. 1986;29:173–178. - PubMed
-
- Robinson SH, Cantoni O, Costa M. Strand breakage and decreased molecular weight of DNA induced by specific metal compunds. Carcinogenesis. 1982;3:657–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
