Aurantoside C Targets and Induces Apoptosis in Triple Negative Breast Cancer Cells
- PMID: 30275391
- PMCID: PMC6213655
- DOI: 10.3390/md16100361
Aurantoside C Targets and Induces Apoptosis in Triple Negative Breast Cancer Cells
Abstract
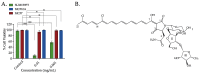
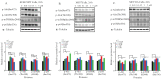
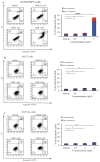
Triple negative breast cancer (TNBC) is a subtype of breast cancers that currently lacks effective targeted therapy. In this study, we found that aurantoside C (C828), isolated from the marine sponge Manihinea lynbeazleyae collected from Western Australia, exhibited higher cytotoxic activities in TNBC cells compared with non-TNBC (luminal and normal-like) cells. The cytotoxic effect of C828 was associated to the accumulation of cell at S-phase, resulting in the decline of cyclin D1, cyclin E1, CDK4, and CDK6, and an increase in p21. We also found that C828 inhibited the phosphorylation of Akt/mTOR and NF-kB pathways and increased the phosphorylation of p38 MAPK and SAPK/JNK pathways, leading to apoptosis in TNBC cells. These effects of C828 were not observed in non-TNBC cells at the concentrations that were cytotoxic to TNBC cells. When compared to the cytotoxic effect with the chemotherapeutic drugs doxorubicin and cisplatin, C828 was found to be 20 times and 35 times more potent than doxorubicin and cisplatin, respectively. These results indicate that C828 could be a promising lead for developing new anticancer agents that target TNBC cells.
Keywords: apoptosis; aurantoside C; cell cycle analysis; marine sponge; triple negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

