Encapsulation of Rat Bone Marrow Derived Mesenchymal Stem Cells in Alginate Dialdehyde/Gelatin Microbeads with and without Nanoscaled Bioactive Glass for In Vivo Bone Tissue Engineering
- PMID: 30275427
- PMCID: PMC6213117
- DOI: 10.3390/ma11101880
Encapsulation of Rat Bone Marrow Derived Mesenchymal Stem Cells in Alginate Dialdehyde/Gelatin Microbeads with and without Nanoscaled Bioactive Glass for In Vivo Bone Tissue Engineering
Abstract
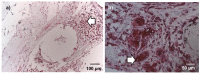
Alginate dialdehyde (ADA), gelatin, and nano-scaled bioactive glass (nBG) particles are being currently investigated for their potential use as three-dimensional scaffolding materials for bone tissue engineering. ADA and gelatin provide a three-dimensional scaffold with properties supporting cell adhesion and proliferation. Combined with nanocristalline BG, this composition closely mimics the mineral phase of bone. In the present study, rat bone marrow derived mesenchymal stem cells (MSCs), commonly used as an osteogenic cell source, were evaluated after encapsulation into ADA-gelatin hydrogel with and without nBG. High cell survival was found in vitro for up to 28 days with or without addition of nBG assessed by calcein staining, proving the cell-friendly encapsulation process. After subcutaneous implantation into rats, survival was assessed by DAPI/TUNEL fluorescence staining. Hematoxylin-eosin staining and immunohistochemical staining for the macrophage marker ED1 (CD68) and the endothelial cell marker lectin were used to evaluate immune reaction and vascularization. After in vivo implantation, high cell survival was found after 1 week, with a notable decrease after 4 weeks. Immune reaction was very mild, proving the biocompatibility of the material. Angiogenesis in implanted constructs was significantly improved by cell encapsulation, compared to cell-free beads, as the implanted MSCs were able to attract endothelial cells. Constructs with nBG showed higher numbers of vital MSCs and lectin positive endothelial cells, thus showing a higher degree of angiogenesis, although this difference was not significant. These results support the use of ADA/gelatin/nBG as a scaffold and of MSCs as a source of osteogenic cells for bone tissue engineering. Future studies should however improve long term cell survival and focus on differentiation potential of encapsulated cells in vivo.
Keywords: alginate dialdehyde; bioactive glass; gelatin; mesenchymal stem cells; nanoparticles; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures







References
-
- Andersen T., Strand B.L., Formo K., Alsberg E., Christensen B.E. Alginates as biomaterials in tissue engineering. Carbohydr. Chem. 2008;37:227–258.
-
- Rokstad A.M., Brekke O.L., Steinkjer B., Ryan L., Kollarikova G., Strand B.L., Skjak-Braek G., Lacik I., Espevik T., Mollnes T.E. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a humand whole blood model. Acta Biomater. 2011;7:2566–2578. doi: 10.1016/j.actbio.2011.03.011. - DOI - PubMed
-
- Veriter S., Mergen J., Goebbels R.M., Aouassar N., Gregoire C., Jordan B., Leveque P., Gallez B., Gianello P., Dufrane D. In vivo selection of biocompatible alginates for islet encapsulation and subcutaneous transplantation. Tissue Eng. Part A. 2010;16:1503–1513. doi: 10.1089/ten.tea.2009.0286. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

