Menstrual cycle rhythmicity: metabolic patterns in healthy women
- PMID: 30275458
- PMCID: PMC6167362
- DOI: 10.1038/s41598-018-32647-0
Menstrual cycle rhythmicity: metabolic patterns in healthy women
Erratum in
-
Publisher Correction: Menstrual cycle rhythmicity: metabolic patterns in healthy women.Sci Rep. 2019 Apr 3;9(1):5797. doi: 10.1038/s41598-019-41392-x. Sci Rep. 2019. PMID: 30940838 Free PMC article.
Abstract
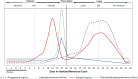
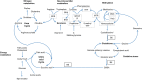
The menstrual cycle is an essential life rhythm governed by interacting levels of progesterone, estradiol, follicular stimulating, and luteinizing hormones. To study metabolic changes, biofluids were collected at four timepoints in the menstrual cycle from 34 healthy, premenopausal women. Serum hormones, urinary luteinizing hormone and self-reported menstrual cycle timing were used for a 5-phase cycle classification. Plasma and urine were analyzed using LC-MS and GC-MS for metabolomics and lipidomics; serum for clinical chemistries; and plasma for B vitamins using HPLC-FLD. Of 397 metabolites and micronutrients tested, 208 were significantly (p < 0.05) changed and 71 reached the FDR 0.20 threshold showing rhythmicity in neurotransmitter precursors, glutathione metabolism, the urea cycle, 4-pyridoxic acid, and 25-OH vitamin D. In total, 39 amino acids and derivatives and 18 lipid species decreased (FDR < 0.20) in the luteal phase, possibly indicative of an anabolic state during the progesterone peak and recovery during menstruation and the follicular phase. The reduced metabolite levels observed may represent a time of vulnerability to hormone related health issues such as PMS and PMDD, in the setting of a healthy, rhythmic state. These results provide a foundation for further research on cyclic differences in nutrient-related metabolites and may form the basis of novel nutrition strategies for women.
Conflict of interest statement
C.F.D., B.W., A.C., S.M. are employees of the Nestlé Group. Funding to conduct this study was provided by Nestle Institute of Health Sciences.
Figures



References
-
- Quabbe HJ. Chronobiology of growth hormone secretion. Chronobiologia. 1977;4:217–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

