IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells
- PMID: 30275536
- PMCID: PMC6804534
- DOI: 10.1038/s41423-018-0166-0
IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells
Abstract
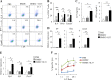
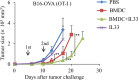
Dendritic cell (DC) tumor vaccines exert their antitumor effects through the induction of effector T cells. We recently identified Tc9 cells as a new potent antitumor effector T cell subset. However, approaches to direct DCs to preferably prime antitumor Tc9 cells should be further exploited. Here, we demonstrate that the addition of interleukin (IL)-33 potently promotes the induction of Tc9 cells by DCs in vitro and in vivo. IL-33 treatment also drives the cytotoxic activities of DC-induced Tc9 cells. Notably, IL-33 treatment enhances cell survival and proliferation of DC-primed CD8+ T cells. More importantly, the addition of IL-33 during in vitro priming of tumor-specific Tc9 cells by DCs increases the antitumor capability of Tc9 cells. Mechanistic studies demonstrated that IL-33 treatment inhibits exhaustive CD8+ T cell differentiation by inhibiting PD-1 and 2B4 expression and increasing IL-2 and CD127 (IL-7 receptor-α, IL-7Rα) expression in CD8+ T cells. Finally, the addition of IL-33 further promotes the therapeutic efficacy of DC-based tumor vaccines in the OT-I mouse model. Our study demonstrates the important role of IL-33 in DC-induced Tc9 cell differentiation and antitumor immunity and may have important clinical implications.
Keywords: Cancer immunology; Dendritic cells; Interleukin-33; Tc9.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

