New opportunities for nanoparticles in cancer immunotherapy
- PMID: 30275967
- PMCID: PMC6158870
- DOI: 10.1186/s40824-018-0133-y
New opportunities for nanoparticles in cancer immunotherapy
Abstract
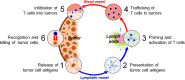
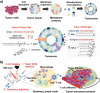
Background: Recently, cancer immunotherapy has become standard for cancer treatment. Immunotherapy not only treats primary tumors, but also prevents metastasis and recurrence, representing a major advantage over conventional cancer treatments. However, existing cancer immunotherapies have limited clinical benefits because cancer antigens are often not effectively delivered to immune cells. Furthermore, unlike lymphoma, solid tumors evade anti-cancer immunity by forming an immune-suppressive tumor microenvironment (TME). One approach for overcoming these limitations of cancer immunotherapy involves nanoparticles based on biomaterials.
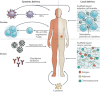
Main body: Here, we review in detail recent trends in the use of nanoparticles in cancer immunotherapy. First, to illustrate the unmet needs for nanoparticles in this field, we describe the mechanisms underlying cancer immunotherapy. We then explain the role of nanoparticles in the delivery of cancer antigens and adjuvants. Next, we discuss how nanoparticles can be helpful within the immune-suppressive TME. Finally, we summarize current and future uses of nanoparticles with image-guided interventional techniques in cancer immunotherapy.
Conclusion: Recently developed approaches for using nanoparticles in cancer immunotherapy have enormous potential for improving cancer treatment. Cancer immunotherapy based on nanoparticles is anticipated not only to overcome the limitations of existing immunotherapy, but also to generate synergistic effects via cooperation between nanoparticles and immune cells.
Keywords: Biomaterials; Cancer antigens; Cancer immunotherapy; Nanoparticle; Tumor microenvironment (TME).
Conflict of interest statement
Not applicableNot applicableThe authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

