Ex Vivo Major Histocompatibility Complex I Knockdown Prolongs Rejection-free Allograft Survival
- PMID: 30276052
- PMCID: PMC6157929
- DOI: 10.1097/GOX.0000000000001825
Ex Vivo Major Histocompatibility Complex I Knockdown Prolongs Rejection-free Allograft Survival
Abstract
Background: Widespread application of vascularized composite allotransplantation (VCA) is currently limited by the required lifelong systemic immunosuppression and its associated morbidity and mortality. This study evaluated the efficacy of ex vivo (after procurement but before transplantation) engineering of allografts using small interfering RNA to knockdown major histocompatibility complex I (MHC-I) and prolong rejection-free survival.
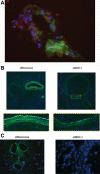
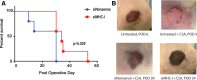
Methods: Endothelial cells (ECs) were transfected with small interfering RNA targeted against MHC-I (siMHC-I) for all in vitro experiments. MHC-I surface expression and knockdown duration were evaluated using quantitative polymerase chain reaction (qPCR) and flow cytometry. After stimulating Lewis recipient cytotoxic lymphocytes (CTL) with allogeneic controls or siMHC-I-silenced ECs, lymphocyte proliferation, CTL-mediated and natural killer-mediated EC lysis were measured. Using an established VCA rat model, allografts were perfused ex vivo with siMHC-I before transplantation. Allografts were analyzed for MHC-I expression and clinical/histologic evidence of rejection.
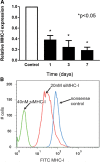
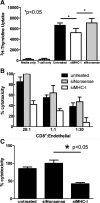
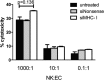
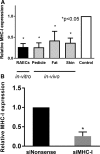
Results: Treatment with siMHC-I resulted in 80% knockdown of mRNA and 87% reduction in cell surface expression for up to 7 days in vitro (P < 0.05). Treatment of ECs with siMHC-I reduced lymphocyte proliferation and CTL-mediated cytotoxicity (77% and 50%, respectively, P < 0.01), without increasing natural killer-mediated cytotoxicity (P = 0.66). In a rat VCA model, ex vivo perfusion with siMHC-I reduced expression in all tissue compartments by at least 50% (P < 0.05). Knockdown prolonged rejection-free survival by 60% compared with nonsense-treated controls (P < 0.05).
Conclusions: Ex vivo siMHC-I engineering can effectively modify allografts and significantly prolong rejection-free allograft survival. This novel approach may help reduce future systemic immunosuppression requirements in VCA recipients.
Figures
Similar articles
-
Ex-vivo treatment of allografts using adipose-derived stem cells induced prolonged rejection-free survival in an allogenic hind-limb transplantation model.Ann Transl Med. 2020 Jul;8(14):867. doi: 10.21037/atm-19-4730. Ann Transl Med. 2020. PMID: 32793711 Free PMC article.
-
Ex vivo transfer of adenovirus-mediated CTLA4Ig gene combined with a short course of rapamycin therapy prolongs free flap allograft survival.Plast Reconstr Surg. 2011 May;127(5):1820-1829. doi: 10.1097/PRS.0b013e31820cf264. Plast Reconstr Surg. 2011. PMID: 21532411
-
Desensitization and Prevention of Antibody-Mediated Rejection in Vascularized Composite Allotransplantation by Syngeneic Hematopoietic Stem Cell Transplantation.Transplantation. 2018 Apr;102(4):593-600. doi: 10.1097/TP.0000000000002070. Transplantation. 2018. PMID: 29298238
-
Large Animal Models of Vascularized Composite Allotransplantation: A Review of Immune Strategies to Improve Allograft Outcomes.Front Immunol. 2021 Jun 30;12:664577. doi: 10.3389/fimmu.2021.664577. eCollection 2021. Front Immunol. 2021. PMID: 34276656 Free PMC article. Review.
-
Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration.Transpl Int. 2016 Jun;29(6):655-62. doi: 10.1111/tri.12652. Epub 2015 Sep 14. Transpl Int. 2016. PMID: 26265179 Free PMC article. Review.
Cited by
-
Ex vivo limb perfusion for traumatic amputation in military medicine.Mil Med Res. 2020 Apr 26;7(1):21. doi: 10.1186/s40779-020-00250-y. Mil Med Res. 2020. PMID: 32334640 Free PMC article.
-
Noninvasive Monitoring of Allograft Rejection Using a Novel Epidermal Sampling Technique.Plast Reconstr Surg Glob Open. 2019 Aug 8;7(8):e2368. doi: 10.1097/GOX.0000000000002368. eCollection 2019 Aug. Plast Reconstr Surg Glob Open. 2019. PMID: 31592385 Free PMC article.
References
-
- Shores JT, Brandacher G, Lee WP. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg. 2015;135:351e. - PubMed
-
- Sosin M, Rodriguez ED. The face transplantation update: 2016. Plast Reconstr Surg. 2016;137:1841. - PubMed
-
- Cetrulo CL, Jr, Li K, Salinas HM, et al. Penis transplantation: first US experience. Ann Surg. 2018;267:983. - PubMed
-
- Bateman C. World’s first successful penis transplant at Tygerberg hospital. S Afr Med J. 2015;105:251. - PubMed
-
- Selvaggi G, Levi DM, Cipriani R, et al. Abdominal wall transplantation: surgical and immunologic aspects. Transplant Proc. 2009;41:521. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
