Tau filaments from multiple cases of sporadic and inherited Alzheimer's disease adopt a common fold
- PMID: 30276465
- PMCID: PMC6208733
- DOI: 10.1007/s00401-018-1914-z
Tau filaments from multiple cases of sporadic and inherited Alzheimer's disease adopt a common fold
Abstract
The ordered assembly of tau protein into abnormal filaments is a defining characteristic of Alzheimer's disease (AD) and other neurodegenerative disorders. It is not known if the structures of tau filaments vary within, or between, the brains of individuals with AD. We used a combination of electron cryo-microscopy (cryo-EM) and immuno-gold negative-stain electron microscopy (immuno-EM) to determine the structures of paired helical filaments (PHFs) and straight filaments (SFs) from the frontal cortex of 17 cases of AD (15 sporadic and 2 inherited) and 2 cases of atypical AD (posterior cortical atrophy). The high-resolution structures of PHFs and SFs from the frontal cortex of 3 cases of AD, 2 sporadic and 1 inherited, were determined by cryo-EM. We also used immuno-EM to study the PHFs and SFs from a number of cortical and subcortical brain regions. PHFs outnumbered SFs in all AD cases. By cryo-EM, PHFs and SFs were made of two C-shaped protofilaments with a combined cross-β/β-helix structure, as described previously for one case of AD. The higher resolution structures obtained here showed two additional amino acids at each end of the protofilament. The immuno-EM findings, which indicated the presence of repeats 3 and 4, but not of the N-terminal regions of repeats 1 and 2, of tau in the filament cores of all AD cases, were consistent with the cryo-EM results. These findings show that there is no significant variation in tau filament structures between individuals with AD. This knowledge will be crucial for understanding the mechanisms that underlie tau filament formation and for developing novel diagnostics and therapies.
Keywords: Alzheimer’s disease; Electron cryo-microscopy; Immuno-gold negative-stain electron microscopy; Neurodegenerative diseases; Paired helical filaments; Straight filaments; Tau protein.
Figures

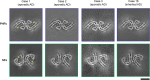
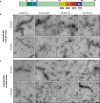

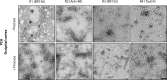
References
-
- Brion J-P, Passareiro H, Nunez J, Flament-Durand J. Mise en évidence immunologique de la protéine tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Arch Biol (Bruxelles) 1985;95:229–235.
-
- Chen S, McMullan G, Faruqi AR, Murshudov GN, Short JM, Scheres SH, Henderson R. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy. 2013;135:24–35. doi: 10.1016/j.ultramic.2013.06.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

