Scan-specific robust artificial-neural-networks for k-space interpolation (RAKI) reconstruction: Database-free deep learning for fast imaging
- PMID: 30277269
- PMCID: PMC6258345
- DOI: 10.1002/mrm.27420
Scan-specific robust artificial-neural-networks for k-space interpolation (RAKI) reconstruction: Database-free deep learning for fast imaging
Abstract
Purpose: To develop an improved k-space reconstruction method using scan-specific deep learning that is trained on autocalibration signal (ACS) data.
Theory: Robust artificial-neural-networks for k-space interpolation (RAKI) reconstruction trains convolutional neural networks on ACS data. This enables nonlinear estimation of missing k-space lines from acquired k-space data with improved noise resilience, as opposed to conventional linear k-space interpolation-based methods, such as GRAPPA, which are based on linear convolutional kernels.
Methods: The training algorithm is implemented using a mean square error loss function over the target points in the ACS region, using a gradient descent algorithm. The neural network contains 3 layers of convolutional operators, with 2 of these including nonlinear activation functions. The noise performance and reconstruction quality of the RAKI method was compared with GRAPPA in phantom, as well as in neurological and cardiac in vivo data sets.
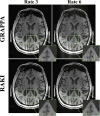
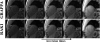
Results: Phantom imaging shows that the proposed RAKI method outperforms GRAPPA at high (≥4) acceleration rates, both visually and quantitatively. Quantitative cardiac imaging shows improved noise resilience at high acceleration rates (rate 4:23% and rate 5:48%) over GRAPPA. The same trend of improved noise resilience is also observed in high-resolution brain imaging at high acceleration rates.
Conclusion: The RAKI method offers a training database-free deep learning approach for MRI reconstruction, with the potential to improve many existing reconstruction approaches, and is compatible with conventional data acquisition protocols.
Keywords: accelerated imaging; convolutional neural networks; deep learning; image reconstruction; k-space interpolation; nonlinear estimation; parallel imaging.
© 2018 International Society for Magnetic Resonance in Medicine.
Figures







References
-
- Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997;38(4):591–603. - PubMed
-
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. - PubMed
-
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. - PubMed
-
- Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28(2):202–213. - PubMed
Publication types
MeSH terms
Grants and funding
- CAREER CCF-1651825/National Science Foundation/International
- P41 EB027061/EB/NIBIB NIH HHS/United States
- P50 NS098573/NS/NINDS NIH HHS/United States
- U01 EB025144/EB/NIBIB NIH HHS/United States
- P30NS076408/NH/NIH HHS/United States
- P41 EB015894/EB/NIBIB NIH HHS/United States
- P41EB015894/NH/NIH HHS/United States
- R01NS085188/NH/NIH HHS/United States
- R00HL111410/NH/NIH HHS/United States
- P30 NS076408/NS/NINDS NIH HHS/United States
- U01EB025144/NH/NIH HHS/United States
- R01 NS085188/NS/NINDS NIH HHS/United States
- CCF-1651825/National Science Foundation/International
- P50NS098573/NH/NIH HHS/United States
- R00 HL111410/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

