Tenascin-X in amniotic fluid and reproductive tissues of pregnancies complicated by infection and preterm prelabor rupture of membranes†
- PMID: 30277495
- PMCID: PMC6437262
- DOI: 10.1093/biolre/ioy216
Tenascin-X in amniotic fluid and reproductive tissues of pregnancies complicated by infection and preterm prelabor rupture of membranes†
Abstract
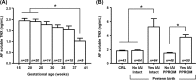
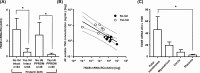
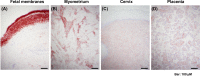
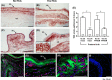
Preterm prelabor rupture of membranes (PPROM), which can precede or follow intra-amniotic infection/inflammation (IAI), is a poorly understood pregnancy complication. Tenascin-X (TNX) is a connective tissue extracellular matrix protein that regulates fibrillogenesis of collagens I, III, and V. Our goal was to investigate the presence and level of soluble TNX (sTNX) in amniotic fluid (AF) and TNX expression in reproductive tissues of pregnancies complicated by PPROM and IAI. We prospectively recruited 334 women pregnant with singletons who had a clinically indicated amniocentesis for genetic karyotyping, lung maturity testing, or rule-out IAI in the presence or absence of PPROM. We quantified TNX expression in fetal membranes, myometrium, cervix, and placenta using immunological methods and qRT-PCR. In pregnancies with normal outcomes, AF sTNX levels were GA-regulated with lower levels toward term. IAI significantly upregulated AF sTNX levels independent of membrane status. AF sTNX levels inversely correlated with fetal membranes tenascin XB (TNXB) mRNA level, which was significantly downregulated by IAI. Western blotting identified characteristic ∼75 and ∼140 kDa sTNX forms in both AF and fetal membranes. Fetal membranes, placenta, and cervix constitutively express TNX with the highest abundance in the amnion. Amnion TNX richness is significantly lost in the setting of IAI. Our results suggest that fetal membranes may be a source of AF sTNX whereby protein and mRNA expression seem to be significantly impacted by inflammation independent of fetal membrane status. A more thorough understanding of TNX changes may be valuable for understanding spontaneous PPROM and to potentially develop therapeutic targets.
Keywords: collagen; fetal membranes; infection; labor; pregnancy.
© The Author(s) 2018. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures

Similar articles
-
Amniotic fluid fetal hemoglobin in normal pregnancies and pregnancies complicated with preterm labor or prelabor rupture of membranes.J Matern Fetal Neonatal Med. 2009 May;22(5):388-97. doi: 10.1080/14767050802578285. J Matern Fetal Neonatal Med. 2009. PMID: 19529995 Free PMC article.
-
Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation.J Matern Fetal Neonatal Med. 2009 Dec;22(12):1151-66. doi: 10.3109/14767050903019684. J Matern Fetal Neonatal Med. 2009. PMID: 19916713 Free PMC article.
-
Pentraxin 3 in Noninvasively Obtained Cervical Fluid Samples from Pregnancies Complicated by Preterm Prelabor Rupture of Membranes.Fetal Diagn Ther. 2019;46(6):402-410. doi: 10.1159/000499482. Epub 2019 May 9. Fetal Diagn Ther. 2019. PMID: 31071711
-
Preterm prelabor rupture of the membranes: A disease of the fetal membranes.Semin Perinatol. 2017 Nov;41(7):409-419. doi: 10.1053/j.semperi.2017.07.012. Epub 2017 Aug 12. Semin Perinatol. 2017. PMID: 28807394 Free PMC article. Review.
-
Matrix metalloproteinases in preterm prelabor rupture of membranes in the setting of chorioamnionitis: A scoping review.Am J Reprod Immunol. 2023 Jan;89(1):e13642. doi: 10.1111/aji.13642. Epub 2022 Nov 9. Am J Reprod Immunol. 2023. PMID: 36300889 Free PMC article.
Cited by
-
The Role of Innate Immune System in the Human Amniotic Membrane and Human Amniotic Fluid in Protection Against Intra-Amniotic Infections and Inflammation.Front Immunol. 2021 Oct 21;12:735324. doi: 10.3389/fimmu.2021.735324. eCollection 2021. Front Immunol. 2021. PMID: 34745106 Free PMC article. Review.
-
Molecular signatures of labor and nonlabor myometrium with parsimonious classification from 2 calcium transporter genes.JCI Insight. 2021 Jun 8;6(11):e148425. doi: 10.1172/jci.insight.148425. JCI Insight. 2021. PMID: 33945511 Free PMC article.
References
-
- Maymon E, Chaim W, Sheiner E, Mazor M. A review of randomized clinical trials of antibiotic therapy in preterm premature rupture of the membranes. Arch Gynecol Obstet 1998; 261:173–181. - PubMed
-
- Mercer BM, Lewis R. Preterm labor and preterm premature rupture of the membranes. Diagnosis and management. Infect Dis Clin North Am 1997; 11:177–201. - PubMed
-
- Polettini J, Dutta EH, Behnia F, Saade GR, Torloni MR, Menon R. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: A systematic review of the literature. Placenta 2015; 36:969–973. - PubMed
-
- Kumar D, Moore RM, Mercer BM, Mansour JM, Redline RW, Moore JJ. The physiology of fetal membrane weakening and rupture: Insights gained from the determination of physical properties revisited. Placenta 2016; 42:59–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

