E2β stimulates ovine uterine artery endothelial cell H2S production in vitro by estrogen receptor-dependent upregulation of cystathionine β-synthase and cystathionine γ-lyase expression†
- PMID: 30277497
- PMCID: PMC6378861
- DOI: 10.1093/biolre/ioy207
E2β stimulates ovine uterine artery endothelial cell H2S production in vitro by estrogen receptor-dependent upregulation of cystathionine β-synthase and cystathionine γ-lyase expression†
Abstract
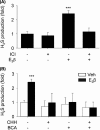
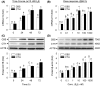
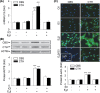
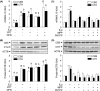
Endogenous hydrogen sulfide (H2S) is a potent vasodilator and proangiogenic second messenger synthesized from L-cysteine by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH). Estrogens are potent vasodilators that stimulate H2S biosynthesis in uterine arteries (UA) in vivo; however, the underlying mechanisms are unknown. We hypothesized that estrogens stimulate H2S biosynthesis in UA endothelial cells (UAEC) via specific estrogen receptor (ER)-dependent mechanisms. In cultured primary UAEC, treatment with estradiol-17β (E2β) stimulated CBS and CTH mRNAs and proteins in a time- and concentration-dependent fashion. As little as 0.1 nM E2β was effective in increasing CBS and CTH expressions and these stimulatory effects maximized with 10-100 nM E2β at 48-72 h. E2β also activated CBS and CTH promoters in UAEC, leading to CBS and CTH expression. Treatment with E2β stimulated H2S production, which was blocked by specific inhibitors of either CBS or CTH and their combination and the ER antagonist ICI 182780. Treatment with either specific agonist of ERα or ERβ stimulated both CBS and CTH mRNA and protein expressions and H2S production to levels similar to that of E2β. Specific antagonist of either ERα or ERβ blocked E2β-stimulated CBS and CTH mRNA and protein expressions and H2S production. Combinations of either ERα or ERβ agonists or their antagonists had no additive effects. Thus, E2β stimulates H2S production by upregulating CBS and CTH mRNA and protein expressions through specific ERα or ERβ-dependent CBS and CTH transcription in UAEC in vitro.
Keywords: endothelium; estrogen; estrogen receptors; hydrogen sulfide; uterine artery; vasodilation.
© The Author(s) 2018. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures
Similar articles
-
ICI 182,780 Attenuates Selective Upregulation of Uterine Artery Cystathionine β-Synthase Expression in Rat Pregnancy.Int J Mol Sci. 2023 Sep 21;24(18):14384. doi: 10.3390/ijms241814384. Int J Mol Sci. 2023. PMID: 37762687 Free PMC article.
-
ERα/ERβ-directed CBS transcription mediates E2β-stimulated hUAEC H2S production.J Mol Endocrinol. 2023 Jan 9;70(2):e220175. doi: 10.1530/JME-22-0175. Print 2023 Feb 1. J Mol Endocrinol. 2023. PMID: 36476832 Free PMC article.
-
Estradiol-17β stimulates H2 S biosynthesis by ER-dependent CBS and CSE transcription in uterine artery smooth muscle cells in vitro.J Cell Physiol. 2019 Jun;234(6):9264-9273. doi: 10.1002/jcp.27606. Epub 2018 Oct 14. J Cell Physiol. 2019. PMID: 30317617 Free PMC article.
-
Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-β- synthase (CBS) and cystathionine-γ-lyase (CSE).Inflamm Allergy Drug Targets. 2011 Apr;10(2):85-91. doi: 10.2174/187152811794776286. Inflamm Allergy Drug Targets. 2011. PMID: 21275900 Review.
-
The Role of the Hydrogen Sulfide Pathway in Male and Female Urogenital System in Health and Disease.Antioxid Redox Signal. 2017 Oct 1;27(10):654-668. doi: 10.1089/ars.2017.7079. Epub 2017 May 19. Antioxid Redox Signal. 2017. PMID: 28398118 Review.
Cited by
-
Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition.Biomolecules. 2020 Apr 30;10(5):697. doi: 10.3390/biom10050697. Biomolecules. 2020. PMID: 32365821 Free PMC article. Review.
-
Neurobehavioral dysfunction in a mouse model of Down syndrome: upregulation of cystathionine β-synthase, H2S overproduction, altered protein persulfidation, synaptic dysfunction, endoplasmic reticulum stress, and autophagy.Geroscience. 2024 Oct;46(5):4275-4314. doi: 10.1007/s11357-024-01146-8. Epub 2024 Apr 1. Geroscience. 2024. PMID: 38558215 Free PMC article.
-
Follicular homocysteine as a marker of oocyte quality in PCOS and the role of micronutrients.J Assist Reprod Genet. 2023 Aug;40(8):1933-1941. doi: 10.1007/s10815-023-02847-3. Epub 2023 Jun 10. J Assist Reprod Genet. 2023. PMID: 37300649 Free PMC article. Clinical Trial.
-
Whole-Genome Uterine Artery Transcriptome Profiling and Alternative Splicing Analysis in Rat Pregnancy.Int J Mol Sci. 2020 Mar 18;21(6):2079. doi: 10.3390/ijms21062079. Int J Mol Sci. 2020. PMID: 32197362 Free PMC article.
-
ICI 182,780 Attenuates Selective Upregulation of Uterine Artery Cystathionine β-Synthase Expression in Rat Pregnancy.Int J Mol Sci. 2023 Sep 21;24(18):14384. doi: 10.3390/ijms241814384. Int J Mol Sci. 2023. PMID: 37762687 Free PMC article.
References
-
- O’Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem 1991; 37:667–672. - PubMed
-
- Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev 1990; 11:124–150. - PubMed
-
- Rosenfeld CR. Responses of reproductive and nonreproductive tissues to 17 beta-estradiol during ovine puerperium. Am J Physiol 1980; 239:E333–E339. - PubMed
-
- Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol 1989; 256:E536–E542. - PubMed
-
- Magness RR, Rosenfeld CR. The role of steroid hormones in the control of uterine blood flow. In: The Uterine Circulation. Ithaca, NY: Perinatology Press; 1989; 10:239–271.

