Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression
- PMID: 30277536
- PMCID: PMC6422439
- DOI: 10.1093/neuonc/noy160
Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression
Abstract
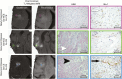
Background: Noninvasively differentiating therapy-induced pseudoprogression from recurrent disease in patients with glioblastoma is prospectively difficult due to the current lack of a biologically specific imaging metric. Ferumoxytol iron oxide nanoparticle MRI contrast characterizes innate immunity mediated neuroinflammation; therefore, we hypothesized that combined ferumoxytol and gadolinium enhanced MRI could serve as a biomarker of glioblastoma pseudoprogression.
Methods: In this institutional review board-approved, retrospective study, we analyzed ferumoxytol and gadolinium contrast enhanced T1-weighted 3T MRI in 45 patients with glioblastoma over multiple clinical timepoints. Isocitrate dehydrogenase 1 (IDH-1) mutational status was characterized by exome sequencing. Sum of products diameter measurements were calculated according to Response Assessment in Neuro-Oncology criteria from both gadolinium and ferumoxytol enhanced sequences. Enhancement mismatch was calculated as the natural log of the ferumoxytol to gadolinium sum of products diameter ratio. Analysis of variance and Student's t-test assessed differences in mismatch ratios. P-value <0.05 indicated statistical significance.
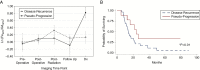
Results: With the development of pseudoprogression we observed a significantly elevated mismatch ratio compared with disease recurrence (P < 0.01) within IDH-1 wild type patients. Patients with IDH-1 mutation demonstrated significantly reduced mismatch ratio with the development of pseudoprogression compared with disease recurrence (P < 0.01). Receiver operator curve analysis demonstrated 100% sensitivity and specificity for the use of mismatch ratios as a diagnostic biomarker of pseudoprogression.
Conclusion: Our study suggests that ferumoxytol to gadolinium contrast mismatch ratios are an MRI biomarker for the diagnosis of pseudoprogression in patients with glioblastoma. This may be due to the unique characterization of therapy-induced neuroinflammation.
Keywords: RANO; ferumoxytol; glioblastoma; macrophage; pseudoprogression.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2018.
Figures


Similar articles
-
Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study.Int J Radiat Oncol Biol Phys. 2011 Feb 1;79(2):514-23. doi: 10.1016/j.ijrobp.2009.10.072. Epub 2010 Apr 13. Int J Radiat Oncol Biol Phys. 2011. PMID: 20395065 Free PMC article.
-
Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question.Neuro Oncol. 2014 Aug;16(8):1146-54. doi: 10.1093/neuonc/not328. Epub 2014 Feb 11. Neuro Oncol. 2014. PMID: 24523362 Free PMC article.
-
Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival.Radiology. 2013 Mar;266(3):842-52. doi: 10.1148/radiol.12111472. Epub 2012 Nov 30. Radiology. 2013. PMID: 23204544 Free PMC article. Clinical Trial.
-
How to stop using gadolinium chelates for magnetic resonance imaging: clinical-translational experiences with ferumoxytol.Pediatr Radiol. 2022 Feb;52(2):354-366. doi: 10.1007/s00247-021-05098-5. Epub 2021 May 27. Pediatr Radiol. 2022. PMID: 34046709 Free PMC article. Review.
-
Pseudoprogression versus true progression in glioblastoma patients: A multiapproach literature review. Part 2 - Radiological features and metric markers.Crit Rev Oncol Hematol. 2021 Mar;159:103230. doi: 10.1016/j.critrevonc.2021.103230. Epub 2021 Jan 27. Crit Rev Oncol Hematol. 2021. PMID: 33515701 Review.
Cited by
-
Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor.Adv Drug Deliv Rev. 2022 Dec;191:114619. doi: 10.1016/j.addr.2022.114619. Epub 2022 Nov 11. Adv Drug Deliv Rev. 2022. PMID: 36372301 Free PMC article. Review.
-
How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy.Am J Cancer Res. 2019 Aug 1;9(8):1546-1553. eCollection 2019. Am J Cancer Res. 2019. PMID: 31497342 Free PMC article. Review.
-
The Emerging Applications of Nanotechnology in Neuroimaging: A Comprehensive Review.Front Bioeng Biotechnol. 2022 Jul 6;10:855195. doi: 10.3389/fbioe.2022.855195. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35875504 Free PMC article. Review.
-
Comparative in vitro and in vivo Evaluation of Different Iron Oxide-Based Contrast Agents to Promote Clinical Translation in Compliance with Patient Safety.Int J Nanomedicine. 2023 Apr 21;18:2071-2086. doi: 10.2147/IJN.S402320. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37113796 Free PMC article.
-
Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends.Pharmaceutics. 2021 Jun 24;13(7):943. doi: 10.3390/pharmaceutics13070943. Pharmaceutics. 2021. PMID: 34202604 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Mirimanoff R, Stupp R. Long-term survival in glioblastoma possible. Updated results of the EORTC/NCIC phase III randomized trial on radiotherapy (RT) and concomitant and adjuvant temozolomide (TMZ) versus RT alone. Int J Radiat Oncol Biol Phys. 2007;69:S2.
-
- Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

