Outcomes in intermediate-risk pediatric lymphocyte-predominant Hodgkin lymphoma: A report from the Children's Oncology Group
- PMID: 30277639
- PMCID: PMC6192844
- DOI: 10.1002/pbc.27375
Outcomes in intermediate-risk pediatric lymphocyte-predominant Hodgkin lymphoma: A report from the Children's Oncology Group
Abstract
Purpose: Optimal management of patients with intermediate-risk lymphocyte-predominant Hodgkin lymphoma (LPHL) is unclear due to their small numbers in most clinical trials. Children's Oncology Group AHOD0031, a randomized phase III trial of pediatric patients with intermediate-risk Hodgkin lymphoma (HL), included patients with LPHL. We report the outcomes of these patients and present directions for future therapeutic strategies.
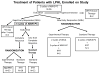
Procedure: Patients received two cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) followed by response evaluation. Slow early responders were randomized to two additional ABVE-PC cycles ± two dexamethasone, etoposide, cisplatin, and cytarabine cycles and all received involved field radiotherapy (IFRT). Rapid early responders (RERs) received two additional ABVE-PC cycles. RERs with complete response (CR) were randomized to IFRT or no further therapy. RERs without CR received IFRT.
Results: Ninety-six (5.6%) of 1711 patients on AHOD0031 had LPHL. Patients with LPHL were more likely to achieve RER (93.6% vs. 81.0%; P = 0.002) and CR (74.2% vs. 49.3%; P = 0.000005) following chemotherapy compared with patients with classical HL. Five-year event-free survival (EFS) was superior in patients with LPHL (92.2%) versus classical HL (83.5%) (P = 0.04), without difference in overall survival (OS). Among RERs with CR following chemotherapy (n = 33), there was no difference in EFS or OS between those randomized to receive or not receive IFRT.
Conclusion: Children and adolescents with intermediate-risk LPHL represent ideal candidates for response-adapted therapy based on their favorable outcomes. The majority of patients treated with the ABVE-PC backbone achieve RER with CR status and can be treated successfully without IFRT.
Keywords: clinical trial; lymphocyte-predominant Hodgkin lymphoma; pediatrics.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures



References
-
- Shankar AG, Ashley S, Radford M, Barrett A, Wright D, Pinkerton CR. Does histology influence outcome in childhood Hodgkin’s disease? Results from the United Kingdom Children’s Cancer Study Group. J Clin Oncol. 1997;15(7):2622–2630. - PubMed
-
- Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20(18):3765–3771. - PubMed
-
- Schwartz CL. Special issues in pediatric Hodgkin’s disease. Eur J Haematol Suppl. 2005;(66):55–62. - PubMed
-
- Punnett A, Tsang RW, Hodgson DC. Hodgkin lymphoma across the age spectrum: epidemiology, therapy, and late effects. Semin Radiat Oncol. 2010;20(1):30–44. - PubMed
-
- Sandoval C, Venkateswaran L, Billups C, Slim M, Jayabose S, Hudson MM. Lymphocyte-predominant Hodgkin disease in children. J Pediatr Hematol Oncol. 2002;24(4):269–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

