Electroencephalographic Biomarkers for Treatment Response Prediction in Major Depressive Illness: A Meta-Analysis
- PMID: 30278789
- PMCID: PMC6312739
- DOI: 10.1176/appi.ajp.2018.17121358
Electroencephalographic Biomarkers for Treatment Response Prediction in Major Depressive Illness: A Meta-Analysis
Abstract
Objective: Reducing unsuccessful treatment trials could improve depression treatment. Quantitative EEG (QEEG) may predict treatment response and is being commercially marketed for this purpose. The authors sought to quantify the reliability of QEEG for response prediction in depressive illness and to identify methodological limitations of the available evidence.
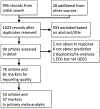
Method: The authors conducted a meta-analysis of diagnostic accuracy for QEEG in depressive illness, based on articles published between January 2000 and November 2017. The review included all articles that used QEEG to predict response during a major depressive episode, regardless of patient population, treatment, or QEEG marker. The primary meta-analytic outcome was the accuracy for predicting response to depression treatment, expressed as sensitivity, specificity, and the logarithm of the diagnostic odds ratio. Raters also judged each article on indicators of good research practice.
Results: In 76 articles reporting 81 biomarkers, the meta-analytic estimates showed a sensitivity of 0.72 (95% CI=0.67-0.76) and a specificity of 0.68 (95% CI=0.63-0.73). The logarithm of the diagnostic odds ratio was 1.89 (95% CI=1.56-2.21), and the area under the receiver operator curve was 0.76 (95% CI=0.71-0.80). No specific QEEG biomarker or specific treatment showed greater predictive power than the all-studies estimate in a meta-regression. Funnel plot analysis suggested substantial publication bias. Most studies did not use ideal practices.
Conclusions: QEEG does not appear to be clinically reliable for predicting depression treatment response, as the literature is limited by underreporting of negative results, a lack of out-of-sample validation, and insufficient direct replication of previous findings. Until these limitations are remedied, QEEG is not recommended for guiding selection of psychiatric treatment.
Keywords: Biological Markers; Mood Disorders-Unipolar; Neurophysiology.
Figures

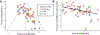
References
-
- Roehrig C Mental disorders top the list of the most costly conditions in the United States: $201 billion. Health Aff (Millwood). 2016. May 18;35(6):1130–5. - PubMed
-
- Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, et al. Practice guideline for the treatment of patients with major depressive disorder third edition. Am J Psychiatry. 2010;167(10):1. - PubMed
-
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006. January 1;163(1):28–40. - PubMed
-
- Rosenblat JD, Lee Y, McIntyre RS. Does pharmacogenomic testing improve clinical outcomes for major depressive disorder? A systematic review of clinical trials and cost-effectiveness studies. J Clin Psychiatry. 2017. June 28;78(6):720–9. - PubMed
-
- Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012. May 2;307(17):1801–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

