Fisetin is a senotherapeutic that extends health and lifespan
- PMID: 30279143
- PMCID: PMC6197652
- DOI: 10.1016/j.ebiom.2018.09.015
Fisetin is a senotherapeutic that extends health and lifespan
Abstract
Background: Senescence is a tumor suppressor mechanism activated in stressed cells to prevent replication of damaged DNA. Senescent cells have been demonstrated to play a causal role in driving aging and age-related diseases using genetic and pharmacologic approaches. We previously demonstrated that the combination of dasatinib and the flavonoid quercetin is a potent senolytic improving numerous age-related conditions including frailty, osteoporosis and cardiovascular disease. The goal of this study was to identify flavonoids with more potent senolytic activity.
Methods: A panel of flavonoid polyphenols was screened for senolytic activity using senescent murine and human fibroblasts, driven by oxidative and genotoxic stress, respectively. The top senotherapeutic flavonoid was tested in mice modeling a progeroid syndrome carrying a p16INK4a-luciferase reporter and aged wild-type mice to determine the effects of fisetin on senescence markers, age-related histopathology, disease markers, health span and lifespan. Human adipose tissue explants were used to determine if results translated.
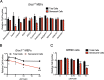
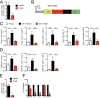
Findings: Of the 10 flavonoids tested, fisetin was the most potent senolytic. Acute or intermittent treatment of progeroid and old mice with fisetin reduced senescence markers in multiple tissues, consistent with a hit-and-run senolytic mechanism. Fisetin reduced senescence in a subset of cells in murine and human adipose tissue, demonstrating cell-type specificity. Administration of fisetin to wild-type mice late in life restored tissue homeostasis, reduced age-related pathology, and extended median and maximum lifespan.
Interpretation: The natural product fisetin has senotherapeutic activity in mice and in human tissues. Late life intervention was sufficient to yield a potent health benefit. These characteristics suggest the feasibility to translation to human clinical studies. FUND: NIH grants P01 AG043376 (PDR, LJN), U19 AG056278 (PDR, LJN, WLL), R24 AG047115 (WLL), R37 AG013925 (JLK), R21 AG047984 (JLK), P30 DK050456 (Adipocyte Subcore, JLK), a Glenn Foundation/American Federation for Aging Research (AFAR) BIG Award (JLK), Glenn/AFAR (LJN, CEB), the Ted Nash Long Life and Noaber Foundations (JLK), the Connor Group (JLK), Robert J. and Theresa W. Ryan (JLK), and a Minnesota Partnership Grant (AMAY-UMN#99)-P004610401-1 (JLK, EAA).
Keywords: Aging; Healthspan; Lifespan; Progeria; Senescence; Senolytic.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



Comment in
-
Geroscience and the Coronavirus Pandemic: The Whack-a-Mole Approach is not Enough.J Am Geriatr Soc. 2020 May;68(5):951-952. doi: 10.1111/jgs.16489. Epub 2020 Apr 27. J Am Geriatr Soc. 2020. PMID: 32293024 Free PMC article. No abstract available.
References
-
- Zhu Y., Armstrong J.L., Tchkonia T., Kirkland J.L. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

