Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility
- PMID: 30279173
- PMCID: PMC6328333
- DOI: 10.1158/2159-8290.CD-18-0193
Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility
Abstract
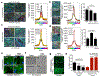
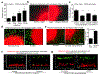
Physical changes in skin are among the most visible signs of aging. We found that young dermal fibroblasts secrete high levels of extracellular matrix (ECM) constituents, including proteoglycans, glycoproteins, and cartilage-linking proteins. The most abundantly secreted was HAPLN1, a hyaluronic and proteoglycan link protein. HAPLN1 was lost in aged fibroblasts, resulting in a more aligned ECM that promoted metastasis of melanoma cells. Reconstituting HAPLN1 inhibited metastasis in an aged microenvironment, in 3-D skin reconstruction models, and in vivo. Intriguingly, aged fibroblast-derived matrices had the opposite effect on the migration of T cells, inhibiting their motility. HAPLN1 treatment of aged fibroblasts restored motility of mononuclear immune cells, while impeding that of polymorphonuclear immune cells, which in turn affected regulatory T-cell recruitment. These data suggest that although age-related physical changes in the ECM can promote tumor cell motility, they may adversely affect the motility of some immune cells, resulting in an overall change in the immune microenvironment. Understanding the physical changes in aging skin may provide avenues for more effective therapy for older patients with melanoma. SIGNIFICANCE: These data shed light on the mechanochemical interactions that occur between aged skin, tumor, and immune cell populations, which may affect tumor metastasis and immune cell infiltration, with implications for the efficacy of current therapies for melanoma.See related commentary by Marie and Merlino, p. 19.This article is highlighted in the In This Issue feature, p. 1.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures



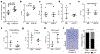
Comment in
-
Ageing matrix promotes metastasis.Nat Rev Cancer. 2018 Dec;18(12):721. doi: 10.1038/s41568-018-0079-3. Nat Rev Cancer. 2018. PMID: 30390019 No abstract available.
-
Something Old, Something New: The Tumor Microenvironment Comes of Age.Cancer Discov. 2019 Jan;9(1):19-21. doi: 10.1158/2159-8290.CD-18-1320. Cancer Discov. 2019. PMID: 30626604 Free PMC article.
References
-
- Balch CM, Thompson JF, Gershenwald JE, Soong SJ, Ding S, McMasters KM, et al. Age as a predictor of sentinel node metastasis among patients with localized melanoma: an inverse correlation of melanoma mortality and incidence of sentinel node metastasis among young and old patients. Ann Surg Oncol 2014;21:1075–81. - PMC - PubMed
-
- Tsai S, Balch C, Lange J. Epidemiology and treatment of melanoma in elderly patients. Nat Rev Clin Oncol 2010;7:148–52. - PubMed
-
- Situm M, Buljan M, Cavka V, Bulat V, Krolo I, Mihic LL. Skin changes in the elderly people--how strong is the influence of the UV radiation on skin aging? Coll Antropol. 2010;34 Suppl 2:9–13. - PubMed
-
- Diridollou S, Vabre V, Berson M, Vaillant L, Black D, Lagarde JM, et al. Skin ageing: changes of physical properties of human skin in vivo. International journal of cosmetic science. 2001;23:353–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA208012/CA/NCI NIH HHS/United States
- R01 CA113451/CA/NCI NIH HHS/United States
- P01 CA114046/CA/NCI NIH HHS/United States
- R01 CA174746/CA/NCI NIH HHS/United States
- R50 CA211199/CA/NCI NIH HHS/United States
- P50 CA174523/CA/NCI NIH HHS/United States
- R01 CA207935/CA/NCI NIH HHS/United States
- U01 CA227550/CA/NCI NIH HHS/United States
- K00 CA212437/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- R50 CA221838/CA/NCI NIH HHS/United States
- F99 CA212437/CA/NCI NIH HHS/United States
- R01 CA131582/CA/NCI NIH HHS/United States
- T32 CA009171/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

