Inhibitory Anti-Peroxidasin Antibodies in Pulmonary-Renal Syndromes
- PMID: 30279272
- PMCID: PMC6218858
- DOI: 10.1681/ASN.2018050519
Inhibitory Anti-Peroxidasin Antibodies in Pulmonary-Renal Syndromes
Abstract
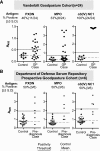
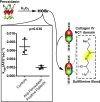
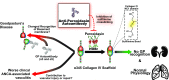
Background: Goodpasture syndrome (GP) is a pulmonary-renal syndrome characterized by autoantibodies directed against the NC1 domains of collagen IV in the glomerular and alveolar basement membranes. Exposure of the cryptic epitope is thought to occur via disruption of sulfilimine crosslinks in the NC1 domain that are formed by peroxidasin-dependent production of hypobromous acid. Peroxidasin, a heme peroxidase, has significant structural overlap with myeloperoxidase (MPO), and MPO-ANCA is present both before and at GP diagnosis in some patients. We determined whether autoantibodies directed against peroxidasin are also detected in GP.
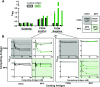
Methods: We used ELISA and competitive binding assays to assess the presence and specificity of autoantibodies in serum from patients with GP and healthy controls. Peroxidasin activity was fluorometrically measured in the presence of partially purified IgG from patients or controls. Clinical disease severity was gauged by Birmingham Vasculitis Activity Score.
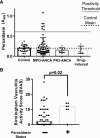
Results: We detected anti-peroxidasin autoantibodies in the serum of patients with GP before and at clinical presentation. Enriched anti-peroxidasin antibodies inhibited peroxidasin-mediated hypobromous acid production in vitro. The anti-peroxidasin antibodies recognized peroxidasin but not soluble MPO. However, these antibodies did crossreact with MPO coated on the polystyrene plates used for ELISAs. Finally, peroxidasin-specific antibodies were also found in serum from patients with anti-MPO vasculitis and were associated with significantly more active clinical disease.
Conclusions: Anti-peroxidasin antibodies, which would previously have been mischaracterized, are associated with pulmonary-renal syndromes, both before and during active disease, and may be involved in disease activity and pathogenesis in some patients.
Keywords: ANCA; Goodpasture-s syndrome; anti-GBM disease; extracellular matrix; glomerulonephritis; vasculitis.
Copyright © 2018 by the American Society of Nephrology.
Figures
Comment in
-
Peroxidasin-a Novel Autoantigen in Anti-GBM Disease?J Am Soc Nephrol. 2018 Nov;29(11):2605-2607. doi: 10.1681/ASN.2018090946. Epub 2018 Oct 12. J Am Soc Nephrol. 2018. PMID: 30314979 Free PMC article. No abstract available.
References
-
- Lee RW, D’Cruz DP: Pulmonary renal vasculitis syndromes. Autoimmun Rev 9: 657–660, 2010 - PubMed
-
- Hudson BG: The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514–2527, 2004 - PubMed
-
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

