In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies
- PMID: 30279409
- PMCID: PMC6168529
- DOI: 10.1038/s41467-018-06086-4
In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies
Erratum in
-
Publisher Correction: In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies.Nat Commun. 2018 Nov 20;9(1):4957. doi: 10.1038/s41467-018-07480-8. Nat Commun. 2018. PMID: 30459411 Free PMC article.
Abstract
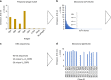
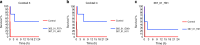
The black mamba (Dendroaspis polylepis) is one of the most feared snake species of the African savanna. It has a potent, fast-acting neurotoxic venom comprised of dendrotoxins and α-neurotoxins associated with high fatality in untreated victims. Current antivenoms are both scarce on the African continent and present a number of drawbacks as they are derived from the plasma of hyper-immunized large mammals. Here, we describe the development of an experimental recombinant antivenom by a combined toxicovenomics and phage display approach. The recombinant antivenom is based on a cocktail of fully human immunoglobulin G (IgG) monoclonal antibodies capable of neutralizing dendrotoxin-mediated neurotoxicity of black mamba whole venom in a rodent model. Our results show the potential use of fully human monoclonal IgGs against animal toxins and the first use of oligoclonal human IgG mixtures against experimental snakebite envenoming.
Conflict of interest statement
The authors declare no competing interests.
Figures



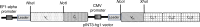
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

