Dye-incorporated coordination polymers for direct photocatalytic trifluoromethylation of aromatics at metabolically susceptible positions
- PMID: 30279417
- PMCID: PMC6168478
- DOI: 10.1038/s41467-018-05919-6
Dye-incorporated coordination polymers for direct photocatalytic trifluoromethylation of aromatics at metabolically susceptible positions
Abstract
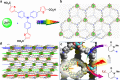
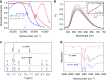
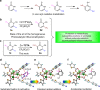
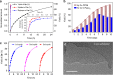
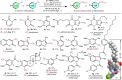
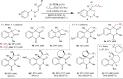
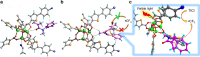
Direct trifluoromethylation of unactivated aromatic rings at metabolically susceptible positions is highly desirable in pharmaceutical applications. By incorporating thiophenes into the backbone of triphenylamine to enlarge its π-system, a new approach for constructing coordination polymers is reported for direct trifluoromethylation without prefunctionalization of the aryl precursors. The improved light-harvesting ability and well-modulated excited state redox potential of the designed polymers endow the generated CF3 radicals with suitable reactivity and enhance radical adduct oxidation in pores. The well-configurated interactions between the organic ligands distort the coordination geometry to create active interaction sites within the coordination polymer; thus, the substrates could be docked near the photoredox-active centres. The synergistic electronic and spatial effects in the confined pores balance the contradictory demands of electronic effects and reaction dynamics, achieving regio- and diastereoselective discrimination among reaction sites with unremarkable electronic/steric differences.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Trifluoromethylation of Arylsilanes with [(phen)CuCF3 ].Angew Chem Int Ed Engl. 2016 Jul 4;55(28):8054-7. doi: 10.1002/anie.201601163. Epub 2016 May 23. Angew Chem Int Ed Engl. 2016. PMID: 27214849 Free PMC article.
-
The mechanism, electronic and ligand effects for reductive elimination from arylPd(II) trifluoromethyl complexes: a systematic DFT study.Dalton Trans. 2015 Mar 14;44(10):4613-22. doi: 10.1039/c4dt03267e. Dalton Trans. 2015. PMID: 25655713
-
Simple and Clean Photoinduced Aromatic Trifluoromethylation Reaction.J Am Chem Soc. 2016 May 11;138(18):5809-12. doi: 10.1021/jacs.6b02782. Epub 2016 May 3. J Am Chem Soc. 2016. PMID: 27137478
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
Direct (hetero)arylation: a new tool for polymer chemists.Acc Chem Res. 2013 Jul 16;46(7):1597-605. doi: 10.1021/ar3003305. Epub 2013 Apr 1. Acc Chem Res. 2013. PMID: 23544354 Review.
Cited by
-
Synergistic photoredox and copper catalysis by diode-like coordination polymer with twisted and polar copper-dye conjugation.Nat Commun. 2020 Oct 23;11(1):5384. doi: 10.1038/s41467-020-19172-3. Nat Commun. 2020. PMID: 33097706 Free PMC article.
-
Rational synthesis of interpenetrated 3D covalent organic frameworks for asymmetric photocatalysis.Chem Sci. 2019 Dec 19;11(6):1494-1502. doi: 10.1039/c9sc04882k. Chem Sci. 2019. PMID: 34084378 Free PMC article.
-
Enantioselective amination of 4-alkylisoquinoline-1,3(2H,4H)-dione derivatives.RSC Adv. 2020 Nov 25;10(70):42912-42915. doi: 10.1039/d0ra07806a. eCollection 2020 Nov 23. RSC Adv. 2020. PMID: 35514906 Free PMC article.
-
Electrochemical Late-Stage Functionalization.Chem Rev. 2023 Oct 11;123(19):11269-11335. doi: 10.1021/acs.chemrev.3c00158. Epub 2023 Sep 26. Chem Rev. 2023. PMID: 37751573 Free PMC article. Review.
-
Fluorescein Hydrazide-Appended Metal-Organic Framework as a Chromogenic and Fluorogenic Chemosensor for Mercury Ions.Molecules. 2021 Sep 23;26(19):5773. doi: 10.3390/molecules26195773. Molecules. 2021. PMID: 34641317 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 21402020/National Natural Science Foundation of China (National Science Foundation of China)/International
- U1608224/National Natural Science Foundation of China (National Science Foundation of China)/International
- U1608224/National Natural Science Foundation of China (National Science Foundation of China)/International
- 21531001/National Natural Science Foundation of China (National Science Foundation of China)/International
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

