Simvastatin Protects Cardiomyocytes Against Endotoxin-induced Apoptosis and Up-regulates Survivin/NF-κB/p65 Expression
- PMID: 30279549
- PMCID: PMC6168467
- DOI: 10.1038/s41598-018-32376-4
Simvastatin Protects Cardiomyocytes Against Endotoxin-induced Apoptosis and Up-regulates Survivin/NF-κB/p65 Expression
Abstract
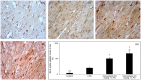
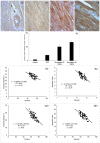
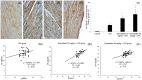
This study is aimed to investigate whether simvastatin induces cardiomyocytes survival signaling in endotoxin (lipopolysaccharide, LSP)-induced myocardial injury, and if so, further to determine a role of survivin in simvastatin-anti-apoptotic effect. Wistar rats were pretreated with simvastatin (10-40 mg/kg po) before a single non-lethal dose of LPS. In myocardial tissue, LPS induced structural disorganization of myofibrils with significant inflammatory infiltrate (cardiac damage score, CDS = 3.87 ± 0.51, p < 0.05), whereas simvastatin dose-dependently abolished structural changes induced by LPS (p < 0.01). Simvastatin in 20 mg/kg and 40 mg/kg pretreatment, dose dependently, attenuated myocardial apoptosis determined as apoptotic index (28.8 ± 4.5% and 18.9 ± 3.5, p < 0.05), decreased cleaved caspase-3 expression (32.1 ± 5.8%, p < 0.01), along with significant Bcl-xL expression in the simvastatin groups (p < 0.01). Interestingly, in the simvastatin groups were determined significantly increased expression of survivin (p < 0.01), but in negative correlation with cleaved caspase-3 and apoptotic indices (p < 0.01). Simvastatin has a cardioprotective effects against LPS induced apoptosis. The effect may be mediated by up-regulation of survivin via activation of NF-κB, which leads to reduced activation of caspase-3 and consequent apoptosis of cardiomyocytes in experimental sepsis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

