Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo
- PMID: 30279727
- PMCID: PMC6160768
- DOI: 10.7150/thno.26975
Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo
Abstract
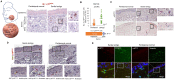
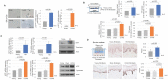
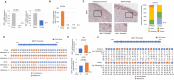
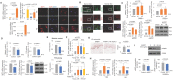
Cutaneous ageing is an important extrinsic process that modifies the pigmentary system. Because cellular senescence is a fundamental ageing mechanism, we examined the role of senescent cells in ageing pigmentation. Methods: Biopsies obtained from senile lentigo and perilesional normal skin were assayed for a marker of cellular senescence, p16INK4A. To determine the secretory phenotypes of senescent fibroblasts, we performed microarray, RNA sequencing and methylation array analyses in senile lentigo and senescent fibroblasts. To further investigate the impact of senescent cells on ageing-related pigmentation, an intervention that targeted senescent cells using radiofrequency was performed. Results:In vivo, senescent fibroblasts accumulated at the sites of age-related pigmentation. Phenotype switching of the cells resulted in the repression of stromal-derived factor 1 (SDF1) by promoter methylation. SDF1 induced melanocyte differentiation via stromal-epithelial interactions, ultimately driving skin pigmentation. Furthermore, the elimination of senescent fibroblasts from pigmented skin using radiofrequency was accompanied by skin lightening, rendering it a potential target for treatment. Conclusion: Aged pigmented skin contains an increasing proportion of senescent fibroblasts. Cells with phenotype switching exhibited a loss of SDF1, which stimulates the melanogenic process and thereby contributes to aging pigmentation. These data may promote the development of new therapeutic paradigms, such as a stroma-targeting therapy for pigmentary disorders.
Keywords: SDF1; Skin pigmentation; senescent fibroblasts; senile lentigo.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Kim M, Han JH, Kim JH, Park TJ, Kang HY. Secreted frizzled-related protein 2 (sFRP2) functions as a melanogenic stimulator; The role of sFRP2 in UV-induced hyperpigmentary disorders. J Invest Dermatol. 2016;136:236–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

