Cancer stem cells in progression of colorectal cancer
- PMID: 30279970
- PMCID: PMC6161799
- DOI: 10.18632/oncotarget.23607
Cancer stem cells in progression of colorectal cancer
Abstract
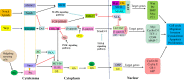
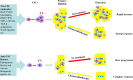
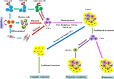
Colorectal cancer is one of the most common cancers worldwide with high mortality. Distant metastasis and relapse are major causes of patient death. Cancer stem cells (CSCs) play a critical role in the metastasis and relapse of colorectal cancer. CSCs are a subpopulation of cancer cells with unique properties of self-renewal, infinite division and multi-directional differentiation potential. Colorectal CSCs are defined with a group of cell surface markers, such as CD44, CD133, CD24, EpCAM, LGR5 and ALDH. They are highly tumorigenic, chemoresistant and radioresistant and thus are critical in the metastasis and recurrence of colorectal cancer and disease-free survival. This review article updates the colorectal CSCs with a focus on their role in tumor initiation, progression, drug resistance and tumor relapse.
Keywords: cancer stem cells; colorectal cancer; epithelial mesenchymal transition; metastasis; tumor microenvironment.
Conflict of interest statement
CONFLICTS OF INTEREST Authors declare no conflicts of interest.
Figures
References
-
- Ozgul Ozdemir RB, Ozdemir AT, Oltulu F, Kurt K, Yigitturk G, Kirmaz C. A comparison of cancer stem cell markers and nonclassical major histocompatibility complex antigens in colorectal tumor and noncancerous tissues. Ann Diagn Pathol. 2016;25:60–3. doi: 10.1016/j.anndiagpath.2016.09.012. - DOI - PubMed
-
- Bu Y, Cao D. The origin of cancer stem cells. Front Biosci (Schol Ed) 2012;4:819–30. - PubMed
-
- Shen Y, Cao D. Hepatocellular carcinoma stem cells: origins and roles in hepatocarcinogenesis and disease progression. Front Biosci (Elite Ed) 2012;4:1157–69. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

