Mapping and Ablation of Idiopathic Ventricular Fibrillation
- PMID: 30280100
- PMCID: PMC6153961
- DOI: 10.3389/fcvm.2018.00123
Mapping and Ablation of Idiopathic Ventricular Fibrillation
Abstract
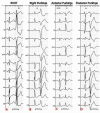
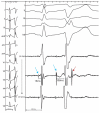
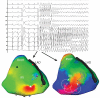
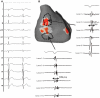
Idiopathic ventricular fibrillation (IVF) is the main cause of unexplained sudden cardiac death, particularly in young patients under the age of 35. IVF is a diagnosis of exclusion in patients who have survived a VF episode without any identifiable structural or metabolic causes despite extensive diagnostic testing. Genetic testing allows identification of a likely causative mutation in up to 27% of unexplained sudden deaths in children and young adults. In the majority of cases, VF is triggered by PVCs that originate from the Purkinje network. Ablation of VF triggers in this setting is associated with high rates of acute success and long-term freedom from VF recurrence. Recent studies demonstrate that a significant subset of IVF defined by negative comprehensive investigations, demonstrate in fact subclinical structural alterations. These localized myocardial alterations are identified by high density electrogram mapping, are of small size and are mainly located in the epicardium. As reentrant VF drivers are often colocated with regions of abnormal electrograms, this localized substrate can be shown to be mechanistically linked with VF. Such areas may represent an important target for ablation.
Keywords: Purkinje; ablation; idiopathic ventricular fibrillation; localized substrate; mapping.
Figures


References
-
- Waldmann V, Bougouin W, Karam N, Dumas F, Sharifzadehgan A, Gandjbakhch E, et al. . Characteristics and clinical assessment of unexplained sudden cardiac arrest in the real-world setting: focus on idiopathic ventricular fibrillation. Eur Heart J. (2018) 39:1981–7. 10.1093/eurheartj/ehy098 - DOI - PMC - PubMed
-
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. (2015) 36:2793–867. 10.1093/eurheartj/ehv316 - DOI - PubMed
-
- Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. . HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm (2013) 10:1932–63. 10.1016/j.hrthm.2013.05.014 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

