Limit to steady-state aerobic power of skeletal muscles
- PMID: 30280281
- PMCID: PMC6208595
- DOI: 10.1007/s10867-018-9510-y
Limit to steady-state aerobic power of skeletal muscles
Abstract
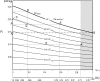
Like any other kind of cell, muscle cells produce energy by oxidizing the fuel substrate that they absorb together with the needed oxygen from the surroundings. Oxidation occurs entirely within the cell. It means that the reactants and products of reaction must at some time be dissolved in the cell's cytosol. If a cell operates at steady state, its cytosol composition remains constant. Therefore, the cytosol in a muscle that produces work at steady state must contain a constant amount of fuel, oxygen, and product of reaction dissolved in it. The greater the power produced, the higher the concentration of these solutes. There is a limit, however, to the maximum amount of solutes that the cytosol can contain without damaging the cell. General thermodynamic arguments, which are reviewed in this paper, help relate this limit to the dehydration and overhydration limits of the cell. The present analysis shows that the same limits entail a limit to the maximum power that a muscle can produce at steady state. This limit depends on the composition of the fuel mixture used by the muscle. The analysis also determines the number of fuel carbon atoms that must be oxidized in parallel within a cell to produce a given power. It may well happen that a muscle cannot reach the maximum attainable power because it cannot activate all the parallel oxidation paths that are needed to produce it. This may be due to a series of reasons ranging from health issues to a lack of training. The paper shows how the methods of indirect calorimetry can provide all the experimental data needed to determine the actual number of parallel oxidation paths that at steady state must be active in a muscle in a given exercise. A diagram relating muscle power to the number of parallel oxidation paths and fuel composition is finally presented. It provides a means to assess the power capacity of animal muscles and can be applied to evaluate their fitness, stamina, margins for improvement, and athletic potential.
Keywords: Aerobic power; Animal power limit; Cell energy production; Muscle power; Steady-state animal power.
Conflict of interest statement
The author declares that he has no conflict of interest.
Figures

Similar articles
-
A comparison of skeletal muscle oxygenation and fuel use in sustained continuous and intermittent exercise.Eur J Appl Physiol Occup Physiol. 1999 Oct;80(5):423-35. doi: 10.1007/s004210050614. Eur J Appl Physiol Occup Physiol. 1999. PMID: 10502076
-
Effect of work and recovery duration on skeletal muscle oxygenation and fuel use during sustained intermittent exercise.Eur J Appl Physiol Occup Physiol. 1999 Oct;80(5):436-47. doi: 10.1007/s004210050615. Eur J Appl Physiol Occup Physiol. 1999. PMID: 10502077
-
Skeletal muscle fuel selection occurs at the mitochondrial level.J Exp Biol. 2014 Jun 1;217(Pt 11):1993-2003. doi: 10.1242/jeb.098863. Epub 2014 Mar 13. J Exp Biol. 2014. PMID: 24625643
-
Does skeletal muscle carnitine availability influence fuel selection during exercise?Proc Nutr Soc. 2018 Feb;77(1):11-19. doi: 10.1017/S0029665117003937. Epub 2017 Oct 17. Proc Nutr Soc. 2018. PMID: 29037265 Review.
-
Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism.Exerc Sport Sci Rev. 1998;26:287-314. Exerc Sport Sci Rev. 1998. PMID: 9696993 Review.
References
-
- Paglietti A. Thermodynamic Limit to the Existence of Inanimate and Living Systems. Milan: Sepco-Acerten; 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

