Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis
- PMID: 30280651
- PMCID: PMC6151253
- DOI: 10.1056/NEJMoa1803484
Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis
Abstract
Background: A vaccine to interrupt the transmission of tuberculosis is needed.
Methods: We conducted a randomized, double-blind, placebo-controlled, phase 2b trial of the M72/AS01E tuberculosis vaccine in Kenya, South Africa, and Zambia. Human immunodeficiency virus (HIV)-negative adults 18 to 50 years of age with latent M. tuberculosis infection (by interferon-γ release assay) were randomly assigned (in a 1:1 ratio) to receive two doses of either M72/AS01E or placebo intramuscularly 1 month apart. Most participants had previously received the bacille Calmette-Guérin vaccine. We assessed the safety of M72/AS01E and its efficacy against progression to bacteriologically confirmed active pulmonary tuberculosis disease. Clinical suspicion of tuberculosis was confirmed with sputum by means of a polymerase-chain-reaction test, mycobacterial culture, or both.
Results: We report the primary analysis (conducted after a mean of 2.3 years of follow-up) of the ongoing trial. A total of 1786 participants received M72/AS01E and 1787 received placebo, and 1623 and 1660 participants in the respective groups were included in the according-to-protocol efficacy cohort. A total of 10 participants in the M72/AS01E group met the primary case definition (bacteriologically confirmed active pulmonary tuberculosis, with confirmation before treatment), as compared with 22 participants in the placebo group (incidence, 0.3 cases vs. 0.6 cases per 100 person-years). The vaccine efficacy was 54.0% (90% confidence interval [CI], 13.9 to 75.4; 95% CI, 2.9 to 78.2; P=0.04). Results for the total vaccinated efficacy cohort were similar (vaccine efficacy, 57.0%; 90% CI, 19.9 to 76.9; 95% CI, 9.7 to 79.5; P=0.03). There were more unsolicited reports of adverse events in the M72/AS01E group (67.4%) than in the placebo group (45.4%) within 30 days after injection, with the difference attributed mainly to injection-site reactions and influenza-like symptoms. Serious adverse events, potential immune-mediated diseases, and deaths occurred with similar frequencies in the two groups.
Conclusions: M72/AS01E provided 54.0% protection for M. tuberculosis-infected adults against active pulmonary tuberculosis disease, without evident safety concerns. (Funded by GlaxoSmithKline Biologicals and Aeras; ClinicalTrials.gov number, NCT01755598 .).
Figures
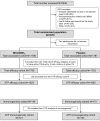
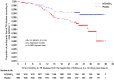
Comment in
-
New Promise for Vaccines against Tuberculosis.N Engl J Med. 2018 Oct 25;379(17):1672-1674. doi: 10.1056/NEJMe1812483. Epub 2018 Sep 25. N Engl J Med. 2018. PMID: 30252629 No abstract available.
-
A vaccine for tuberculosis.Nat Med. 2018 Nov;24(11):1637. doi: 10.1038/s41591-018-0258-5. Nat Med. 2018. PMID: 30401862 No abstract available.
References
-
- World Health Organization. Global Tuberculosis Report 2017. (http://www.who.int/tb/publications/global_report/en/)
-
- Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 2017;Mar 5. - PubMed
-
- Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2014;58:470-80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
