Transplanted Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Support Liver Regeneration in Gunn Rats
- PMID: 30280963
- PMCID: PMC7366275
- DOI: 10.1089/scd.2018.0010
Transplanted Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Support Liver Regeneration in Gunn Rats
Abstract
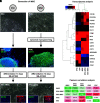
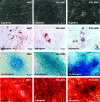
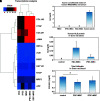
Gunn rats bear a mutation within the uridine diphosphate glucuronosyltransferase-1a1 (Ugt1a1) gene resulting in high serum bilirubin levels as seen in Crigler-Najjar syndrome. In this study, the Gunn rat was used as an animal model for heritable liver dysfunction. Induced mesenchymal stem cells (iMSCs) derived from embryonic stem cells (H1) and induced pluripotent stem cells were transplanted into Gunn rats after partial hepatectomy. The iMSCs engrafted and survived in the liver for up to 2 months. The transplanted iMSCs differentiated into functional hepatocytes as evidenced by partially suppressed hyperbilirubinemia and expression of multiple human-specific hepatocyte markers such as albumin, hepatocyte nuclear factor 4α, UGT1A1, cytokeratin 18, bile salt export pump, multidrug resistance protein 2, Na/taurocholate-cotransporting polypeptide, and α-fetoprotein. These findings imply that transplanted human iMSCs can contribute to liver regeneration in vivo and thus represent a promising tool for the treatment of inherited liver diseases.
Keywords: ESC; fetal MSC; iMSC; iPSC; liver regeneration; stem cell transplantation.
Conflict of interest statement
No competing financial interest exists.
Figures

References
-
- Crigler JF., Jr. and Najjar VA. (1952). Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics 10:169–180 - PubMed
-
- Ruud Hansen TW. (2010). Phototherapy for neonatal jaundice—therapeutic effects on more than one level? Semin Perinatol 34:231–234 - PubMed
-
- Ramy N, Ghany EA, Alsharany W, Nada A, Darwish RK, Rabie WA. and Aly H. (2016). Jaundice, phototherapy and DNA damage in full-term neonates. J Perinatol 36:132–136 - PubMed
-
- van Dijk R, Beuers U. and Bosma PJ. (2015). Gene replacement therapy for genetic hepatocellular jaundice. Clin Rev Allergy Immunol 48:243–253 - PubMed
-
- Jorns C, Nowak G, Nemeth A, Zemack H, Mörk LM, Johansson H, Gramignoli R, Watanabe M, Karadagi A, et al. (2016). De novo donor-specific HLA antibody formation in two patients with Crigler–Najjar Syndrome Type I following human hepatocyte transplantation with partial hepatectomy preconditioning. Am J Transplant 16:1021–1030 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

