Acetalated Dextran Microparticles for Codelivery of STING and TLR7/8 Agonists
- PMID: 30281314
- PMCID: PMC6261357
- DOI: 10.1021/acs.molpharmaceut.8b00579
Acetalated Dextran Microparticles for Codelivery of STING and TLR7/8 Agonists
Abstract
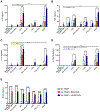
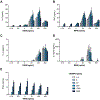
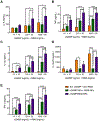
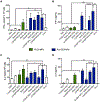
Vaccines are the most effective tool for preventing infectious diseases; however, subunit vaccines, considered the safest type, suffer from poor immunogenicity and require adjuvants to create a strong and sustained immune response. As adjuvants, pathogen-associated molecular patterns (PAMPs) offer potent immunostimulatory properties and defined mechanisms of action through their cognate pattern recognition receptors (PRRs). Their activity can be further enhanced through combining two or more PAMPs, particularly those that activate multiple immune signaling pathways. However, the cytosolic localization of many PRRs requires intracellular delivery of PAMPs for optimal biological activity, which is particularly true of the stimulator of interferon genes (STING) PRR. Using acetalated dextran (Ace-DEX) microparticles (MPs) encapsulating STING agonist 3'3'-cyclic GMP-AMP (cGAMP) combined with soluble PAMPS, we screened the effect of codelivery of adjuvants using primary mouse bone marrow derived dendritic cells (BMDCs). We identified that codelivery of cGAMP MPs and soluble Toll-like receptor 7/8 (TLR7/8) agonist resiquimod (R848) elicited the broadest cytokine response. cGAMP and R848 were then coencapsulated within Ace-DEX MPs via electrospray. Using the model antigen ovalbumin, we observed that Ace-DEX MPs coencapsulating cGAMP and R848 (cGAMP/R848 Ace-DEX MPs) induced antigen-specific cellular immunity, and a balanced Th1/Th2 humoral response that was greater than cGAMP Ace-DEX MPs alone and PAMPs delivered in separate MPs. These data indicate that polymeric Ace-DEX MPs loaded with STING and TLR7/8 agonists represent a potent cellular and humoral vaccine adjuvant.
Keywords: STING; acetalated dextran; cGAMP adjuvant; microparticles; vaccine adjuvants.
Conflict of interest statement
The authors declare the following competing financial interest(s): Drs. Ainslie, Ting, and Bachelder serve on the advisory board for IMMvention Therapeutix, Inc. Although a financial conflict of interest was identified for management based on the overall scope of the project and its potential benefit to IMMvention Therapeutix, Inc., the research findings included in the publication may not necessarily relate to the interests of IMMvention Therapeutix, Inc. The terms of this arrangement have been reviewed by the University of North Carolina at Chapel Hill in accordance with its policy on objectivity in research.
Figures




Similar articles
-
A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination.J Control Release. 2018 Jan 28;270:1-13. doi: 10.1016/j.jconrel.2017.11.030. Epub 2017 Nov 21. J Control Release. 2018. PMID: 29170142 Free PMC article.
-
Multi-COBRA hemagglutinin formulated with cGAMP microparticles elicits protective immune responses against influenza viruses.mSphere. 2024 Jul 30;9(7):e0016024. doi: 10.1128/msphere.00160-24. Epub 2024 Jun 26. mSphere. 2024. PMID: 38920382 Free PMC article.
-
Investigation of tunable acetalated dextran microparticle platform to optimize M2e-based influenza vaccine efficacy.J Control Release. 2018 Nov 10;289:114-124. doi: 10.1016/j.jconrel.2018.09.020. Epub 2018 Sep 24. J Control Release. 2018. PMID: 30261204 Free PMC article.
-
Delivery of STING agonists for adjuvanting subunit vaccines.Adv Drug Deliv Rev. 2021 Dec;179:114020. doi: 10.1016/j.addr.2021.114020. Epub 2021 Oct 29. Adv Drug Deliv Rev. 2021. PMID: 34756942 Review.
-
The use of Toll-like receptor 7/8 agonists as vaccine adjuvants.Expert Rev Vaccines. 2013 Jul;12(7):809-19. doi: 10.1586/14760584.2013.811208. Expert Rev Vaccines. 2013. PMID: 23885825 Review.
Cited by
-
Tumor Activated Cell Penetrating Peptides to Selectively Deliver Immune Modulatory Drugs.Pharmaceutics. 2021 Mar 10;13(3):365. doi: 10.3390/pharmaceutics13030365. Pharmaceutics. 2021. PMID: 33801967 Free PMC article.
-
Influenza Virus and SARS-CoV-2 Vaccines.J Immunol. 2021 Jun 1;206(11):2509-2520. doi: 10.4049/jimmunol.2001287. Epub 2021 May 21. J Immunol. 2021. PMID: 34021048 Free PMC article. Review.
-
Bioinspired vaccines to enhance MHC class-I antigen cross-presentation.Curr Opin Immunol. 2022 Aug;77:102215. doi: 10.1016/j.coi.2022.102215. Epub 2022 Jun 4. Curr Opin Immunol. 2022. PMID: 35667222 Free PMC article. Review.
-
Amphiphilic Polyelectrolyte Graft Copolymers Enhance the Activity of Cyclic Dinucleotide STING Agonists.Adv Healthc Mater. 2021 Jan;10(2):e2001056. doi: 10.1002/adhm.202001056. Epub 2020 Nov 23. Adv Healthc Mater. 2021. PMID: 33225632 Free PMC article.
-
Immunomodulation as a Novel Strategy for Prevention and Treatment of Bordetella spp. Infections.Front Immunol. 2019 Dec 13;10:2869. doi: 10.3389/fimmu.2019.02869. eCollection 2019. Front Immunol. 2019. PMID: 31921136 Free PMC article. Review.
References
-
- Pliaka V; Kyriakopoulou Z; Markoulatos P Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis. Expert Rev. Vaccines 2012, 11 (5), 609–28. - PubMed
-
- Minor PD Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–92. - PubMed
-
- Riese P; Schulze K; Ebensen T; Prochnow B; Guzman CA Vaccine adjuvants: key tools for innovative vaccine design. Curr. Top. Med. Chem 2013, 13 (20), 2562–80. - PubMed
-
- O’Hagan DT; Ott GS; De Gregorio E; Seubert A The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine 2012, 30 (29), 4341–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

