Elastase Exocytosis by Airway Neutrophils Is Associated with Early Lung Damage in Children with Cystic Fibrosis
- PMID: 30281324
- PMCID: PMC6444666
- DOI: 10.1164/rccm.201803-0442OC
Elastase Exocytosis by Airway Neutrophils Is Associated with Early Lung Damage in Children with Cystic Fibrosis
Abstract
Rationale: Neutrophils are recruited to the airways of individuals with cystic fibrosis (CF). In adolescents and adults with CF, airway neutrophils actively exocytose the primary granule protease elastase (NE), whose extracellular activity correlates with lung damage. During childhood, free extracellular NE activity is measurable only in a subset of patients, and the exocytic function of airway neutrophils is unknown.
Objectives: To measure NE exocytosis by airway neutrophils in relation to free extracellular NE activity and lung damage in children with CF.
Methods: We measured lung damage using chest computed tomography coupled with the Perth-Rotterdam Annotated Grid Morphometric Analysis for Cystic Fibrosis scoring system. Concomitantly, we phenotyped blood and BAL fluid leukocytes by flow and image cytometry, and measured free extracellular NE activity using spectrophotometric and Förster resonance energy transfer assays. Children with airway inflammation linked to aerodigestive disorder were enrolled as control subjects.
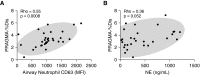
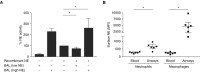
Measurements and main results: Children with CF but not disease control children harbored BAL fluid neutrophils with high exocytosis of primary granules, before the detection of bronchiectasis. This measure of NE exocytosis correlated with lung damage (R = 0.55; P = 0.0008), whereas the molecular measure of free extracellular NE activity did not. This discrepancy may be caused by the inhibition of extracellular NE by BAL fluid antiproteases and its binding to leukocytes.
Conclusions: NE exocytosis by airway neutrophils occurs in all children with CF, and its cellular measure correlates with early lung damage. These findings implicate live airway neutrophils in early CF pathogenesis, which should instruct biomarker development and antiinflammatory therapy in children with CF.
Keywords: air trapping; degranulation; mucus plugging; proteolysis; scavenging.
Figures


Comment in
-
Letting It All Out: Neutrophils in Early Cystic Fibrosis Airway Inflammation.Am J Respir Crit Care Med. 2019 Apr 1;199(7):816-817. doi: 10.1164/rccm.201810-1951ED. Am J Respir Crit Care Med. 2019. PMID: 30395720 Free PMC article. No abstract available.
References
-
- Grasemann H, Ratjen F. Early lung disease in cystic fibrosis. Lancet Respir Med. 2013;1:148–157. - PubMed
-
- VanDevanter DR, Kahle JS, O'Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros. 2016;15:147–157. - PubMed
-
- Ranganathan SC, Hall GL, Sly PD, Stick SM, Douglas TA Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Early lung disease in infants and preschool children with cystic fibrosis. What have we learned and what should we do about it? Am J Respir Crit Care Med. 2017;195:1567–1575. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

