Eprobe mediated RT-qPCR for the detection of leukemia-associated fusion genes
- PMID: 30281597
- PMCID: PMC6169845
- DOI: 10.1371/journal.pone.0202429
Eprobe mediated RT-qPCR for the detection of leukemia-associated fusion genes
Abstract
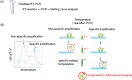
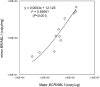
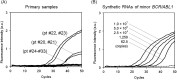
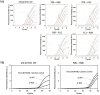
The detection and quantification of leukemia-associated fusion gene transcripts play important roles in the diagnosis and follow-up of leukemias. To establish a standardized method without interlaboratory discrepancies, we developed a novel one-step reverse transcription quantitative PCR (RT-qPCR) assay, called "the Eprobe leukemia assay," for major and minor BCR-ABL1, RUNX1-RUNX1T1, and various isoforms of PML-RARA. This assay is comprised of Eprobes that are exciton-controlled hybridization-sensitive fluorescent oligonucleotides. Melting curve analyses were performed on synthetic quantitative standard RNAs with strict quality control. Quantification capacity was evaluated by comparison with TaqMan RT-qPCR using 67 primary leukemia patient samples. The lower limit of detection and the limit of quantification of this assay were less than 31.3 copies/reaction and 62.5 copies/reaction, respectively. This assay correctly detected the fusion genes in samples with 100% sensitivity and specificity. The specificity of the reactions was confirmed by melting curve analyses. The assay detected low-level expression of minor BCR-ABL1 co-expressed with major BCR-ABL1. These results illustrate the feasibility and high accuracy of the Eprobe leukemia assay, even for minimal residual disease monitoring.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Cazzaniga G, Gaipa G, Rossi V, Biondi A. Minimal residual disease as a surrogate marker for risk assignment to ALL patients. Rev Clin Exp Hematol. 2003;7(3):292–323. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

