Evaluation of a set of refolded recombinant antigens for serodiagnosis of human fascioliasis
- PMID: 30281608
- PMCID: PMC6169862
- DOI: 10.1371/journal.pone.0203490
Evaluation of a set of refolded recombinant antigens for serodiagnosis of human fascioliasis
Abstract
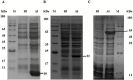
Diagnosis of fascioliasis with high sensitivity and specificity antigens play a vital role in the management of the disease. Majority of commercial serological tests use F. hepatica native antigens and indicate wide diversities in test accuracy. Nowadays, recombinant antigens have been introduced as diagnostic reagents offer better test standardization. A combination of highly pure recombinant antigens associated with correct folding will leads to improve specificity and sensitivity of ELISA for diagnosis of Fascioliasis. In this article, Fasciola hepatica saposin-like protein 2 (SAP-2), ferritin protein (Ftn-1) and leucine aminopeptidase (LAP) recombinant antigens were considered as tools for the detection of F. hepatica immunoglobulin G antibodies in persons with chronic human fasciolasis. The recombinant antigens were obtained as fusion proteins, expressed in Escherichia coli and purified by immobilized metal affinity chromatography (IMAC). The refolding processes of denatured recombinant proteins were performed using dialysis method in the presence of chemical additives, and reduced/oxidized glutathione (in vitro). The immunoreactivity of the recombinant antigens was assessed individually and in a combination compared with excretory/secretory antigen (E/S) in an enzyme-linked immunosorbent assay (ELISA) test. The experiments were optimized using 213 serum samples from humans, including patients with chronic fascioliasis, patients with other parasitic diseases, and healthy subjects. The results indicated 95% sensitivity and 98% specificity for rtFhSAP-2, 96% sensitivity and 91% specificity for E/S, 80% and 83.3% for rtFhFtn-1, 84% and 88% for FhLAP, and also, 96% and 95% for combination of recombinant antigens, respectively. In conclusion, the results of this investigation showed that rtFhSAP-2 with the highest specificity and acceptable sensitivity has a considerable superiority compared to mentioned antigens and even in combination with these antigens in serodiagnosis of human fascioliasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Recent Developments in Recombinant Proteins for Diagnosis of Human Fascioliasis.Acta Parasitol. 2021 Mar;66(1):13-25. doi: 10.1007/s11686-020-00280-5. Epub 2020 Sep 24. Acta Parasitol. 2021. PMID: 32974849 Review.
-
Expression, purification and in vitro refolding of the recombinant truncated Saposin-like protein 2 antigen for development of diagnosis of human fascioliasis.Acta Trop. 2017 Jul;171:163-171. doi: 10.1016/j.actatropica.2017.03.002. Epub 2017 Mar 11. Acta Trop. 2017. PMID: 28300559
-
Comparative assessment of recombinant and native immunogenic forms of Fasciola hepatica proteins for serodiagnosis of sheep fasciolosis.Parasitol Res. 2018 Jan;117(1):225-232. doi: 10.1007/s00436-017-5696-3. Epub 2017 Dec 4. Parasitol Res. 2018. PMID: 29199372
-
Designing and Developing Serological Test for the Diagnosis of Human Fascioliasis Using a New Recombinant Multi-epitope.Acta Parasitol. 2024 Mar;69(1):1005-1015. doi: 10.1007/s11686-024-00796-0. Epub 2024 Mar 18. Acta Parasitol. 2024. PMID: 38498251
-
Fas2-ELISA in the detection of human infection by Fasciola hepatica.J Helminthol. 2005 Sep;79(3):235-40. doi: 10.1079/joh2005303. J Helminthol. 2005. PMID: 16153317 Review.
Cited by
-
Recent Developments in Recombinant Proteins for Diagnosis of Human Fascioliasis.Acta Parasitol. 2021 Mar;66(1):13-25. doi: 10.1007/s11686-020-00280-5. Epub 2020 Sep 24. Acta Parasitol. 2021. PMID: 32974849 Review.
-
Immunoproteomic Approach of Extracellular Antigens From Paracoccidioides Species Reveals Exclusive B-Cell Epitopes.Front Microbiol. 2020 Jan 28;10:2968. doi: 10.3389/fmicb.2019.02968. eCollection 2019. Front Microbiol. 2020. PMID: 32117076 Free PMC article.
References
-
- Organization WH. Report of the WHO Informal Meeting on use of triclabendazole in fascioliasis control. WHO headquarters, Geneva, Switzerland, 17–18 October 2006. World Health Organization, Geneva ISBN. 2007;92(4):154638.
-
- Mas‐Coma S, Angles R, Esteban J, Bargues M, Buchon P, Franken M, et al. The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Tropical Medicine & International Health. 1999;4(6):454–67. - PubMed
-
- Mas-Coma S, Bargues M, Esteban J. Human fasciolosis. Fasciolosis. 1999;411:434.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

