Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer
- PMID: 30281875
- PMCID: PMC6215885
- DOI: 10.1111/cas.13813
Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer
Abstract
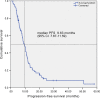
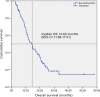
The present study is the first phase II clinical trial aimed to evaluate the efficacy and safety of S-1 plus nanoparticle albumin-bound paclitaxel (Nab-PTX) as first-line chemotherapy for advanced gastric cancer (AGC). Previously untreated patients with metastatic gastric adenocarcinoma received S-1 in oral doses of 40 mg (BSA <1.25 m2 ), 50 mg (1.25 ≤ BSA < 1.50 m2 ) and 60 mg (BSA ≥1.50 m2 ) b.i.d. on days 1-14 in combination with Nab-PTX (120 mg/m2 , on days 1 and 8) for each 21-day cycle. Primary endpoint was progression-free survival (PFS), and secondary endpoints were overall response rate (ORR), overall survival (OS), disease control rate (DCR), and toxicity. A total of 73 gastric cancer patients with metastatic and measurable lesions were enrolled in the first-line setting. Median PFS and OS were 9.63 months and 14.60 months, respectively. Four (5.5%) patients had complete responses, 39 (53.4%) had partial responses (PRs), 21 (28.8%) had stable disease, four (5.5%) progressed and five (6.8%) were not evaluable. ORR and DCR were 58.9% and 87.7%, respectively. Most toxicities were mild, and no treatment-related deaths occurred. Grade 3 to 4 toxicities occurred in 22 patients (30.1%) as follows: leukopenia (13.7%), neutropenia (12.3%), anemia (5.5%), thrombocytopenia (1.4%), diarrhea (6.8%), vomiting (2.7%), stomatitis (1.4%), peripheral neuropathy (1.4%), and hand-foot syndrome (1.4%). Seven patients achieved good responses and underwent gastrectomy plus metastasectomy. Thirty (41.1%) patients had S-1 maintenance with a median of four cycles. S-1 plus Nab-PTX is an efficient and safe regimen as first-line treatment for patients with AGC.
Keywords: S-1; advanced gastric cancer; chemotherapy; first-line; nanoparticle albumin-bound paclitaxel.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376:687‐697. - PubMed
-
- Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S‐1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5‐fluorouracil in the First‐Line Advanced Gastric Cancer Study. Eur J Can. 2013;49:3616‐3624. - PubMed
-
- Al‐Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435‐1442. - PubMed
-
- Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S‐1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547‐1553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical