Impact of obesity on the toxicity of a multi-ingredient dietary supplement, OxyELITE Pro™ (New Formula), using the novel NZO/HILtJ obese mouse model: Physiological and mechanistic assessments
- PMID: 30282009
- PMCID: PMC6219625
- DOI: 10.1016/j.fct.2018.09.067
Impact of obesity on the toxicity of a multi-ingredient dietary supplement, OxyELITE Pro™ (New Formula), using the novel NZO/HILtJ obese mouse model: Physiological and mechanistic assessments
Abstract
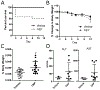
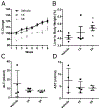
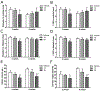
Herbal dietary supplement (HDS)-induced hepato- and cardiotoxicity is an emerging clinical problem. In this study, we investigated the liver and heart toxicity of HDS OxyELITE-PRO™ New Formula (OEP-NF), a dietary supplement marketed for weight loss and performance enhancement that was recently withdrawn from the market. Using a novel NZO/HlLtJ obese mouse model, we demonstrated that administration of clinically relevant mouse equivalent doses (MED) of OEP-NF produced cardio- and hepatotoxic risks following both short- and long-term administration schedules. Specifically, gavaging female NZO/HlLtJ with up to 2X MED of OEP-NF resulted in 40% mortality within two weeks. Feeding mice with either 1X or 3X MED of OEP-NF for eight weeks, while not exhibiting significant effects on body weights, significantly altered hepatic gene expression, increased the number of apoptotic and mast cells in the heart and affected cardiac function. The degree of toxicity in NZO/HlLtJ mice was higher than that observed previously in non-obese CD-1 and B6C3F1 strains, suggesting that an overweight/obese condition can sensitize mice to OEP-NF. Adverse health effects linked to OEP-NF, together with a number of other hepato- and cardiotoxicity cases associated with HDS ingestion, argue strongly for introduction of quality standards and pre-marketing safety assessments for multi-ingredient HDS.
Keywords: Cardiotoxicity; Hepatotoxicity; Herbal-induced liver injury; New dietary ingredient; OXYElite pro; Phytochemicals.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Hepatotoxicity due to herbal dietary supplements: Past, present and the future.Food Chem Toxicol. 2022 Nov;169:113445. doi: 10.1016/j.fct.2022.113445. Epub 2022 Sep 29. Food Chem Toxicol. 2022. PMID: 36183923 Free PMC article. Review.
-
Safety assessment of the dietary supplement OxyELITE™ Pro (New Formula) in inbred and outbred mouse strains.Food Chem Toxicol. 2017 Nov;109(Pt 1):194-209. doi: 10.1016/j.fct.2017.08.025. Epub 2017 Aug 24. Food Chem Toxicol. 2017. PMID: 28843594 Free PMC article.
-
Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro™ - United States, 2013.Drug Test Anal. 2017 Jan;9(1):68-74. doi: 10.1002/dta.2036. Epub 2016 Aug 4. Drug Test Anal. 2017. PMID: 27367536 Free PMC article.
-
Hepatotoxicity associated with the dietary supplement OxyELITE Pro™ - Hawaii, 2013.Drug Test Anal. 2016 Mar-Apr;8(3-4):319-27. doi: 10.1002/dta.1894. Epub 2015 Nov 2. Drug Test Anal. 2016. PMID: 26538199 Free PMC article.
-
Drug-induced Liver Injury Secondary to Herbal and Dietary Supplements.Clin Liver Dis. 2020 Feb;24(1):141-155. doi: 10.1016/j.cld.2019.09.009. Epub 2019 Oct 31. Clin Liver Dis. 2020. PMID: 31753247 Review.
Cited by
-
Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease.Int J Mol Sci. 2022 Jan 19;23(3):1062. doi: 10.3390/ijms23031062. Int J Mol Sci. 2022. PMID: 35162986 Free PMC article. Review.
-
Decaffeinated Green Tea Extract Does Not Elicit Hepatotoxic Effects and Modulates the Gut Microbiome in Lean B6C3F₁ Mice.Nutrients. 2019 Apr 3;11(4):776. doi: 10.3390/nu11040776. Nutrients. 2019. PMID: 30987244 Free PMC article.
-
Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration.Molecules. 2019 Jun 17;24(12):2256. doi: 10.3390/molecules24122256. Molecules. 2019. PMID: 31212965 Free PMC article.
-
Hepatotoxicity due to herbal dietary supplements: Past, present and the future.Food Chem Toxicol. 2022 Nov;169:113445. doi: 10.1016/j.fct.2022.113445. Epub 2022 Sep 29. Food Chem Toxicol. 2022. PMID: 36183923 Free PMC article. Review.
-
Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model.Molecules. 2019 Apr 30;24(9):1694. doi: 10.3390/molecules24091694. Molecules. 2019. PMID: 31052254 Free PMC article.
References
-
- Arseculeratne SN, Gunatilaka AA, Panabokke RG. Studies of medicinal plants of Sri Lanka. Part 14: Toxicity of some traditional medicinal herbs. J Ethnopharmacol. 1985. July;13(3):323–35. - PubMed
-
- Aubert J, Begriche K, Delannoy M, Morel I, Pajaud J, Ribault C, Lepage S, McGill MR, Lucas-Clerc C, Turlin B, Robin MA, Jaeschke H, Fromenty B. Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db mice. J Pharmacol Exp Ther. 2012. September;342(3):676–87. doi: 10.1124/jpet.112.193813. - DOI - PubMed
-
- Avula B, Chittiboyina AG, Wang YH, Sagi S, Raman V, Wang M, Khan IA. Simultaneous Determination of Aegeline and Six Coumarins from Different Parts of the Plant Aegle marmelos Using UHPLC-PDA-MS and Chiral Separation of Aegeline Enantiomers Using HPLC-ToF-MS. Planta Med. 2016. April;82(6):580–8. doi: 10.1055/s-0042-103160. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

