Positive Feedback Loops in Alzheimer's Disease: The Alzheimer's Feedback Hypothesis
- PMID: 30282364
- PMCID: PMC6484277
- DOI: 10.3233/JAD-180583
Positive Feedback Loops in Alzheimer's Disease: The Alzheimer's Feedback Hypothesis
Abstract
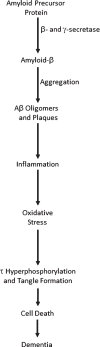
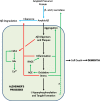
The dominant model for Alzheimer's disease (AD) is the amyloid cascade hypothesis, in which the accumulation of excess amyloid-β (Aβ) leads to inflammation, excess glutamate and intracellular calcium, oxidative stress, tau hyperphosphorylation and tangle formation, neuronal loss, and ultimately dementia. In a cascade, AD proceeds in a unidirectional fashion, with events only affecting downstream processes. Compelling evidence now exists for the presence of positive feedback loops in AD, however, involving oxidative stress, inflammation, glutamate, calcium, and tau. The pathological state of AD is thus a system of positive feedback loops, leading to amplification of the initial perturbation, rather than a linear cascade. Drugs may therefore be effective by targeting numerous points within the loops, rather than concentrating on upstream processes. Anti-inflammatories and anti-oxidants may be especially valuable, since these processes are involved in many loops and hence would affect numerous processes in AD.
Keywords: Aggregation; amyloid; amyloid-β protein precursor; directed acyclic graph; drug discovery; peptide; systems biology.
Conflict of interest statement
The author’s disclosure is available online (
Figures
References
-
- Hardy JA, Higgins GA (1992) Alzheimer’s disease: The amyloid cascade hypothesis. Science 256, 184–185. - PubMed
-
- Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LDB, Moore S, Daria A, Hampel H, Muller V, Giudici C, Nuscher B, Wenninger-Weinzierl A, Kremmer E, Heneka MT, Thal DR, Giedraitis V, Lannfelt L, Muller U, Livesey FJ, Meissner F, Herms J, Konnerth A, Marie H, Haass C (2015) Eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 526, 443. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

