Safety, efficacy, and onset of a novel botulinum toxin type A (Nabota) for the treatment of glabellar frown lines: a single-arm, prospective, phase 4 clinical study
- PMID: 30282425
- PMCID: PMC6177673
- DOI: 10.7181/acfs.2018.01886
Safety, efficacy, and onset of a novel botulinum toxin type A (Nabota) for the treatment of glabellar frown lines: a single-arm, prospective, phase 4 clinical study
Abstract
Background: Safety, efficacy, and time to onset of effect of botulinum toxin type A is of importance to persons who seek improvement in glabellar frown lines, but this has not been well studied. The aim of this study was to determine the safety, efficacy, and onset of action of a newly developed botulinum toxin type A (Nabota) for the treatment of glabellar frown lines.
Methods: This was a single-arm, open-label, and phase 4 clinical study. Forty-two subjects with glabellar lines were treated with five times of intramuscular injection of 0.1 mL (4 U/0.1 mL) for a total of 20 U of Nabota. Efficacy and safety were assessed at 2, 3, 4, 5, and 14 days. Efficacy was assessed by the investigator and it was defined as a 1-point change on a 4-point scale.
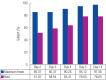
Results: Improvement in glabellar frown lines at maximum frown was observed in 85.4% of subjects 2 days after administration. Improvement in glabellar lines at rest was observed in 51.2% of subjects 2 days after administration, and the proportion of subjects showing improvement increased with time. No severe adverse events were recorded.
Conclusion: Onset of action was observed in the majority of subjects by 2 days after administration of Nabota. In addition, Nabota was found to be safe and effective for the treatment of glabellar frown lines.
Keywords: Botulinum toxins, type A; Efficacy; Glabellar frown line; Onset; Safety.
Conflict of interest statement
Dr. Joon Pio Hong and Dr. Tae Suk Oh serve as consultants and lecturers for Daewoong Pharmaceutical. This study was sponsored by Daewoong Pharmaceutical. The sponsor was involved in the collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. No other potential conflict of interest relevant to this article was reported.
Figures



References
-
- Tsui JK. Botulinum toxin as a therapeutic agent. Pharmacol Ther. 1996;72:13–24. - PubMed
-
- Pearce LB, First ER, MacCallum RD, Gupta A. Pharmacologic characterization of botulinum toxin for basic science and medicine. Toxicon. 1997;35:1373–412. - PubMed
-
- Kim CS, Jang WS, Son IP, Nam SH, Kim YI, Park KY, et al. Electrophysiological study for comparing the effect of biological activity between type A botulinum toxins in rat gastrocnemius muscle. Hum Exp Toxicol. 2013;32:914–20. - PubMed
-
- Honeck P, Weiss C, Sterry W, Rzany B; Gladys study group. Reproducibility of a four-point clinical severity score for glabellar frown lines. Br J Dermatol. 2003;149:306–10. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

