Effects of three-dimensionally printed polycaprolactone/β-tricalcium phosphate scaffold on osteogenic differentiation of adipose tissue- and bone marrow-derived stem cells
- PMID: 30282427
- PMCID: PMC6177683
- DOI: 10.7181/acfs.2018.01879
Effects of three-dimensionally printed polycaprolactone/β-tricalcium phosphate scaffold on osteogenic differentiation of adipose tissue- and bone marrow-derived stem cells
Abstract
Background: Autogenous bone grafts have several limitations including donor-site problems and insufficient bone volume. To address these limitations, research on bone regeneration is being conducted actively. In this study, we investigate the effects of a three-dimensionally (3D) printed polycaprolactone (PCL)/tricalcium phosphate (TCP) scaffold on the osteogenic differentiation potential of adipose tissue-derived stem cells (ADSCs) and bone marrow-derived stem cells (BMSCs).
Methods: We investigated the extent of osteogenic differentiation on the first and tenth day and fourth week after cell culture. Cytotoxicity of the 3D printed PCL/β-TCP scaffold was evaluated by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay, prior to osteogenic differentiation analysis. ADSCs and BMSCs were divided into three groups: C, only cultured cells; M, cells cultured in the 3D printed PCL/β-TCP scaffold; D, cells cultured in the 3D printed PCL/β-TCP scaffold with a bone differentiation medium. Alkaline phosphatase (ALP) activity assay, von Kossa staining, reverse transcription-polymerase chain reaction (RT-PCR), and Western blotting were performed for comparative analysis.
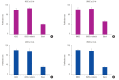
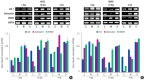
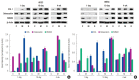
Results: ALP assay and von Kossa staining revealed that group M had higher levels of osteogenic differentiation compared to group C. RT-PCR showed that gene expression was higher in group M than in group C, indicating that, compared to group C, osteogenic differentiation was more extensive in group M. Expression levels of proteins involved in ossification were higher in group M, as per the Western blotting results.
Conclusion: Osteogenic differentiation was increased in mesenchymal stromal cells (MSCs) cultured in the 3D printed PCL/TCP scaffold compared to the control group. Osteogenic differentiation activity of MSCs cultured in the 3D printed PCL/TCP scaffold was lower than that of cells cultured on the scaffold in bone differentiation medium. Collectively, these results indicate that the 3D printed PCL/TCP scaffold promoted osteogenic differentiation of MSCs and may be widely used for bone tissue engineering.
Keywords: Adipose tissue; Bone marrow; Cell differentiation; Mesenchymal stromal cells; Polycaprolactone; Stem cells; Tricalcium phosphate.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




References
-
- Liao HT, Lee MY, Tsai WW, Wang HC, Lu WC. Osteogenesis of adipose-derived stem cells on polycaprolactone-β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J Tissue Eng Regen Med. 2016;10:E337–53. - PubMed
-
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. - PubMed
-
- Rose FR, Cyster LA, Grant DM, Scotchford CA, Howdle SM, Shakesheff KM. In vitro assessment of cell penetration into porous hydroxyapatite scaffolds with a central aligned channel. Biomaterials. 2004;25:5507–14. - PubMed
-
- Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29–39. - PubMed
-
- Arafat MT, Lam CX, Ekaputra AK, Wong SY, Li X, Gibson I. Biomimetic composite coating on rapid prototyped scaffolds for bone tissue engineering. Acta Biomater. 2011;7:809–20. - PubMed
LinkOut - more resources
Full Text Sources
Medical

