Lessons from pressure denaturation of proteins
- PMID: 30282759
- PMCID: PMC6228469
- DOI: 10.1098/rsif.2018.0244
Lessons from pressure denaturation of proteins
Abstract
Although it is now relatively well understood how sequence defines and impacts global protein stability in specific structural contexts, the question of how sequence modulates the configurational landscape of proteins remains to be defined. Protein configurational equilibria are generally characterized by using various chemical denaturants or by changing temperature or pH. Another thermodynamic parameter which is less often used in such studies is high hydrostatic pressure. This review discusses the basis for pressure effects on protein structure and stability, and describes how the unique mechanisms of pressure-induced unfolding can provide unique insights into protein conformational landscapes.
Keywords: pressure; protein folding; protein stability.
© 2018 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures
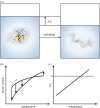


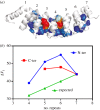
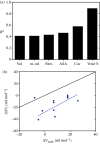
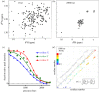
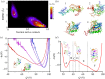
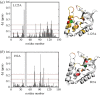
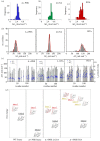

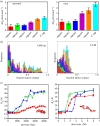

 .
.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
