Cell Cycle Changes after Glioblastoma Stem Cell Irradiation: The Major Role of RAD51
- PMID: 30282933
- PMCID: PMC6213228
- DOI: 10.3390/ijms19103018
Cell Cycle Changes after Glioblastoma Stem Cell Irradiation: The Major Role of RAD51
Abstract
"Glioma Stem Cells" (GSCs) are known to play a role in glioblastoma (GBM) recurrence. Homologous recombination (HR) defects and cell cycle checkpoint abnormalities can contribute concurrently to the radioresistance of GSCs. DNA repair protein RAD51 homolog 1 (RAD51) is a crucial protein for HR and its inhibition has been shown to sensitize GSCs to irradiation. The aim of this study was to examine the consequences of ionizing radiation (IR) for cell cycle progression in GSCs. In addition, we intended to assess the potential effect of RAD51 inhibition on cell cycle progression. Five radiosensitive GSC lines and five GSC lines that were previously characterized as radioresistant were exposed to 4Gy IR, and cell cycle analysis was done by fluorescence-activated cell sorting (FACS) at 24, 48, 72, and 96 h with or without RAD51 inhibitor. Following 4Gy IR, all GSC lines presented a significant increase in G2 phase at 24 h, which was maintained over 72 h. In the presence of RAD51 inhibitor, radioresistant GSCs showed delayed G2 arrest post-irradiation for up to 48 h. This study demonstrates that all GSCs can promote G2 arrest in response to radiation-induced DNA damage. However, following RAD51 inhibition, the cell cycle checkpoint response differed. This study contributes to the characterization of the radioresistance mechanisms of GSCs, thereby supporting the rationale of targeting RAD51-dependent repair pathways in view of radiosensitizing GSCs.
Keywords: DNA repair protein RAD51 homolog 1 (RAD51); Glioma Stem Cells; cell cycle; ionizing radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

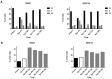


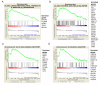
References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-Year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003;63:5821–5828. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

