CPP-Ts: a new intracellular calcium channel modulator and a promising tool for drug delivery in cancer cells
- PMID: 30282983
- PMCID: PMC6170434
- DOI: 10.1038/s41598-018-33133-3
CPP-Ts: a new intracellular calcium channel modulator and a promising tool for drug delivery in cancer cells
Abstract
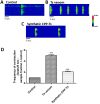
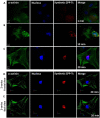
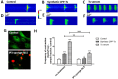
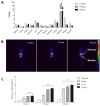
Scorpion sting envenoming impacts millions of people worldwide, with cardiac effects being one of the main causes of death on victims. Here we describe the first Ca2+ channel toxin present in Tityus serrulatus (Ts) venom, a cell penetrating peptide (CPP) named CPP-Ts. We show that CPP-Ts increases intracellular Ca2+ release through the activation of nuclear InsP3R of cardiomyocytes, thereby causing an increase in the contraction frequency of these cells. Besides proposing a novel subfamily of Ca2+ active toxins, we investigated its potential use as a drug delivery system targeting cancer cell nucleus using CPP-Ts's nuclear-targeting property. To this end, we prepared a synthetic CPP-Ts sub peptide14-39 lacking pharmacological activity which was directed to the nucleus of specific cancer cell lines. This research identifies a novel subfamily of Ca2+ active toxins and provides new insights into biotechnological applications of animal venoms.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World health organization (WHO). Rabies and envenomings: a neglected public health issue: report of a consultative meeting. (World Health Organization, Geneva, 36 p. 2007).
-
- Gordon D, Savarin P, Gurevitz M, Zinn-Justin S. Functional anatomy of scorpion toxins affecting sodium channels. J Toxicol Toxin Rev., v. 1998;17:131–159. doi: 10.3109/15569549809009247. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

