Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms
- PMID: 30283025
- PMCID: PMC6170476
- DOI: 10.1038/s41598-018-33016-7
Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms
Abstract
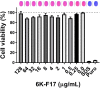
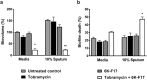
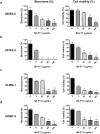
With the increasing recognition of biofilms in human disease, the development of novel antimicrobial therapies is of critical importance. For example, in patients with cystic fibrosis (CF), the acquisition of host-adapted, chronic Pseudomonas aeruginosa infection is associated with a decline in lung function and increased mortality. Our objective was to test the in vitro efficacy of a membrane-active antimicrobial peptide we designed, termed 6K-F17 (sequence: KKKKKK-AAFAAWAAFAA-NH2), against multidrug resistant P. aeruginosa biofilms. This peptide displays high antimicrobial activity against a range of pathogenic bacteria, yet is non-hemolytic to human erythrocytes and non-toxic to human bronchial epithelial cells. In the present work, P. aeruginosa strain PAO1, and four multidrug resistant (MDR) isolates from chronically infected CF individuals, were grown as 48-hour biofilms in a static biofilm slide chamber model. These biofilms were then exposed to varying concentrations of 6K-F17 alone, or in the presence of tobramycin, prior to confocal imaging. Biofilm biovolume and viability were assessed. 6K-F17 was able to kill biofilms - even in the presence of sputum - and greatly reduce biofilm biovolume in PAO1 and MDR isolates. Strikingly, when used in conjunction with tobramycin, low doses of 6K-F17 significantly potentiated tobramycin killing, leading to biofilm destruction.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Engineered cationic antimicrobial peptide (eCAP) prevents Pseudomonas aeruginosa biofilm growth on airway epithelial cells.J Antimicrob Chemother. 2016 Aug;71(8):2200-7. doi: 10.1093/jac/dkw143. Epub 2016 May 26. J Antimicrob Chemother. 2016. PMID: 27231279 Free PMC article.
-
Anti-Infectives Restore ORKAMBI® Rescue of F508del-CFTR Function in Human Bronchial Epithelial Cells Infected with Clinical Strains of P. aeruginosa.Biomolecules. 2020 Feb 19;10(2):334. doi: 10.3390/biom10020334. Biomolecules. 2020. PMID: 32092967 Free PMC article.
-
Effect of L-arginine on cystic fibrosis Pseudomonas aeruginosa biofilms.Antimicrob Agents Chemother. 2024 Aug 7;68(8):e0033624. doi: 10.1128/aac.00336-24. Epub 2024 Jul 18. Antimicrob Agents Chemother. 2024. PMID: 39023260 Free PMC article.
-
Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections.Am J Respir Cell Mol Biol. 2018 Apr;58(4):428-439. doi: 10.1165/rcmb.2017-0321TR. Am J Respir Cell Mol Biol. 2018. PMID: 29372812 Free PMC article. Review.
-
Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients.Adv Drug Deliv Rev. 2015 May;85:7-23. doi: 10.1016/j.addr.2014.11.017. Epub 2014 Dec 2. Adv Drug Deliv Rev. 2015. PMID: 25477303 Review.
Cited by
-
Developing antibacterial peptides as a promising therapy for combating antibiotic-resistant Pseudomonas aeruginosa infections.Vet World. 2024 Jun;17(6):1259-1264. doi: 10.14202/vetworld.2024.1259-1264. Epub 2024 Jun 8. Vet World. 2024. PMID: 39077460 Free PMC article.
-
Antimicrobial Peptides against Multidrug-Resistant Pseudomonas aeruginosa Biofilm from Cystic Fibrosis Patients.J Med Chem. 2022 Jul 14;65(13):9050-9062. doi: 10.1021/acs.jmedchem.2c00270. Epub 2022 Jun 27. J Med Chem. 2022. PMID: 35759644 Free PMC article.
-
Co-expression Mechanism Analysis of Different Tachyplesin I-Resistant Strains in Pseudomonas aeruginosa Based on Transcriptome Sequencing.Front Microbiol. 2022 Apr 7;13:871290. doi: 10.3389/fmicb.2022.871290. eCollection 2022. Front Microbiol. 2022. PMID: 35464984 Free PMC article.
-
Emerging therapies against infections with Pseudomonas aeruginosa.F1000Res. 2019 Aug 7;8:F1000 Faculty Rev-1371. doi: 10.12688/f1000research.19509.1. eCollection 2019. F1000Res. 2019. PMID: 31448090 Free PMC article. Review.
-
Effects of human β-defensin 3 fused with carbohydrate-binding domain on the function of type III secretion system in Pseudomonas aeruginosa PA14.Braz J Microbiol. 2020 Mar;51(1):29-35. doi: 10.1007/s42770-020-00223-2. Epub 2020 Jan 13. Braz J Microbiol. 2020. PMID: 31933178 Free PMC article.
References
-
- Cystic Fibrosis Canada. Canadian Patient Data Registry Report. (Toronto, Canada, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

