Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD)
- PMID: 30283029
- PMCID: PMC6447473
- DOI: 10.1038/s41380-018-0256-5
Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD)
Erratum in
-
Correction: Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD).Mol Psychiatry. 2020 Jul;25(7):1604. doi: 10.1038/s41380-018-0311-2. Mol Psychiatry. 2020. PMID: 30617276 Free PMC article.
Abstract
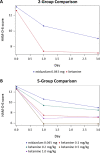
Numerous placebo-controlled studies have demonstrated the ability of ketamine, an NMDA receptor antagonist, to induce rapid (within hours), transient antidepressant effects when administered intravenously (IV) at subanesthetic doses (0.5 mg/kg over 40 min). However, the optimal antidepressant dose remains unknown. We aimed to compare to active placebo the rapid acting antidepressant properties of a broad range of subanesthetic doses of IV ketamine among outpatients with treatment-resistant depression (TRD). A range of IV ketamine doses were compared to active placebo in the treatment of adult TRD over a 3-day period following a single infusion over 40 min. This was an outpatient study conducted across six US academic sites. Outpatients were 18-70 years old with TRD, defined as failure to achieve a satisfactory response (e.g., less than 50% improvement of depression symptoms) to at least two adequate treatment courses during the current depressive episode. Following a washout period, 99 eligible subjects were randomly assigned to one of the five arms in a 1:1:1:1:1 fashion: a single intravenous dose of ketamine 0.1 mg/kg (n = 18), a single dose of ketamine 0.2 mg/kg (n = 20), a single dose of ketamine 0.5 mg/kg (n = 22), a single dose of ketamine 1.0 mg/kg (n = 20), and a single dose of midazolam 0.045 mg/kg (active placebo) (n = 19). The study assessments (HAM-D-6, MADRS, SDQ, PAS, CGI-S, and CGI-I) were performed at days 0, 1, 3 (endpoint), 5, 7, 14, and 30 to assess the safety and efficacy. The overall group × time interaction effect was significant for the primary outcome measure, the HAM-D-6. In post hoc pairwise comparisons controlling for multiple comparisons, standard dose (0.5 mg/kg) and high dose (1 mg/kg) of intravenous ketamine were superior to active placebo; a low dose (0.1 mg/kg) was significant only prior to adjustment (p = 0.02, p-adj = 0.14, d = -0.82 at day 1). Most of the interaction effect was due to differences at day 1, with no significant adjusted pairwise differences at day 3. This pattern generally held for secondary outcomes. The infusions of ketamine were relatively well tolerated compared to active placebo, except for greater dissociative symptoms and transient blood pressure elevations with the higher doses. Our results suggest that there is evidence for the efficacy of the 0.5 mg/kg and 1.0 mg/kg subanesthetic doses of IV ketamine and no clear or consistent evidence for clinically meaningful efficacy of lower doses of IV ketamine. Trial Registration: NCT01920555.
Conflict of interest statement
LCC, MF, BBH, and HJ declare that they have no conflict of interest. CD: Dr. DeBattista has received grant support from Janssen, Neuronetics, St. Jude, and Biolite. He has served on the Advisory Board of Alkermes. CC: Dr. Cusin receives funding from NIMH (R01MH102279) and has received consulting fees from Janssen Pharmaceuticals, Takeda, Boehringer, Lundbeck. She has also participated in research funded by Janssen, Medtronic, Otsuka, Takeda. MF: reports 3-year disclosures as below: all lifetime disclosures can be viewed online at:
Figures
References
-
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. - PubMed
-
- Bauer M, El-Khalili N, Datto C, Szamosi J, Eriksson H. A pooled analysis of two randomised, placebo-controlled studies of extended release quetiapine fumarate adjunctive to antidepressant therapy in patients with major depressive disorder. J Affect Disord. 2010;127:19–30. doi: 10.1016/j.jad.2010.08.032. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical